Abstract
Purpose
Local treatment of uveal melanoma by radiotherapy involves the use of brachytherapy with radioactive plaques attached to the sclera, or proton irradiation. Both treatments induce growth arrest within the tumour and its slow involution over several years. Although ocular retention rates are excellent, regrowth of tumours due to resistance and neovascular glaucoma leads to enucleation of up to 10% of affected eyes. Proton irradiation involves part of the iris in most cases and we noticed that neovascularisation only occurred in the part of the iris that was not irradiated. We therefore conducted this study to determine the relationship between the development of iris neovascularisation and iris irradiation.
Methods
A total of 21 enucleation specimens from patients who had previously had proton irradiation were collected from the files of the Department of Pathology, Moorfields Eye Hospital during the 5-year period from 1994 to 1999. Sections of these eyes were assessed for VEGF-A, bFGF, and von Willebrand Factor (vWF) by immunohistochemistry. Ophthalmic notes and radiotherapy records were reviewed to assess the extent of iris irradiation.
Results
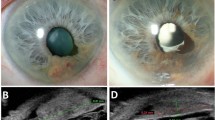
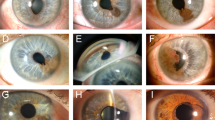
In all, 11 cases showed clinical evidence of iris neovascularisation and were selected for further study. Three of these eyes also showed clinical evidence of regrowth of the tumour. Histological evidence of iris neovascularization was noted in all 11 of the eyes examined, and was only present in the nonirradiated side of the iris in 8/11 eyes. NVI was present on both sides of the iris in three cases, but was less severe in the irradiated part. Expression of VEGF-A was at most weak within the tumour, but was present in the detached retina and in the epithelium of both ciliary body and iris. Some bFGF staining was noted around vessels in the iris stroma.
Conclusions
Our results suggest that irradiation leads to iris atrophy, and that atrophic, irradiated iris is resistant to the development of neovascularisation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gragoudas ES . Proton beam therapy of uveal melanomas. Arch Ophthalmol 1986; 104: 349–351.
Suit HD . Protons to replace photons in external beam radiation therapy? Clin Oncol (R Coll Radiol) 2003; 15: S29–S31.
Chiquet C, Grange JD, Ayzac L, Chauvel P, Patricot LM, Devouassoux-Shisheboran M . Effects of proton beam irradiation on uveal melanomas: a comparative study of Ki-67 expression in irradiated versus non-irradiated melanomas. Br J Ophthalmol 2000; 84: 98–102.
Seddon JM, Gragoudas ES, Albert DM . Ciliary body and choroidal melanomas treated by proton beam irradiation. Histopathologic study of eyes. Arch Ophthalmol 1983; 101: 1402–1408.
Ferry AP, Blair CJ, Gragoudas ES, Volk SC . Pathologic examination of ciliary body melanoma treated with proton beam irradiation. Arch Ophthalmol 1985; 103: 1849–1853.
Kincaid MC, Folberg R, Torczynski E, Zakov ZN, Shore JW, Liu SJ et al. Complications after proton beam therapy for uveal malignant melanoma. A clinical and histopathologic study of five cases. Ophthalmology 1988; 95: 982–991.
Liszauer AD, Brownstein S, Corriveau C, Deschenes J . A clinicopathological study of seven globes enucleated after primary radiation therapy for malignant melanoma of the choroid or ciliary body. Can J Ophthalmol 1990; 25: 340–344.
Saornil MA, Egan KM, Gragoudas ES, Seddon JM, Walsh SM, Albert DM . Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol 1992; 110: 1112–1118.
Char DH, Quivey JM, Castro JR, Kroll S, Phillips T . Helium ions versus iodine 125 brachytherapy in the managment of uveal melanoma. Ophthalmology 1993; 100: 1547–1554.
Foss AJ, Whelehan I, Hungerford JL, Anderson DF, Errington RD, Kacperek A et al. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br J Ophthalmol 1997; 81: 748–754.
Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM . Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol 2002; 120: 1665–1671.
Egger E, Zografos L, Schalenbourg A, Beati D, Bohringer T, Chamot L et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 2003; 55: 867–880.
Bonnett DE, Kacperek A, Sheen MA, Goodall R, Saxton TE . The 62 MeV proton beam for the treatment of ocular melanoma at Clatterbridge. Br J Radiol 1993; 66: 907–914.
Boyd SR, Tan DS, de Souza L, Neale MH, Myatt NE, Alexander RA et al. Uveal melanomas express vascular endothelial growth factor and basic fibroblast growth factor and support endothelial cell growth. Br J Ophthalmol 2002; 86: 440–447.
Sheidow TG, Hooper PL, Crukley C, Young J, Heathcote JG . Expression of vascular endothelial growth factor in uveal melanoma and its correlation with metastasis. Br J Ophthalmol 2000; 84: 750–756.
Coppin JM . Malignant melanoma and rubeosis iridis. Br J Ophthalmol 1973; 57: 815–824.
Gimbrone MA, Leapman SB, Cotran RS, Folkman J . Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumours. J Natl Cancer Inst 1973; 50: 219–228.
Gragoudas ES, Seddon J, Egan K, Glynn R, Munzenrider J, Austin-Seymour M et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology 1987; 94: 349–353.
Boyd SR, Tan D, Bunce C, Gittos A, Neale MH, Hungerford JL et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol 2002a; 86: 448–452.
Brovkina AF, Zarubei GD . Ciliochoroidal melanomas treated with narrow medical proton beam. Arch Ophthalmol 1986; 104: 402–404.
Kim MK, Char DH, Castro JL, Saunders WM, Chen GTY, Stone RD . Neovascular glaucoma after helium ion irradiation for uveal melanoma. Ophthalmology 1986; 93: 189–192.
Lumbroso L, Desjardins L, Levy C, Plancher C, Frau E, D'Hermies F et al. Intraocular inflammation after proton beam irradiation for uveal melanoma. Br J Ophthalmol 2001; 85: 1305–1308.
Constable PH, Crowston JG, Occleston NL, Cordeiro MF, Khaw PT . Long term growth arrest of human Tenon's fibroblasts following single applications of beta radiation. Br J Ophthalmol 1998; 82: 448–452.
Levin LA, Gragoudas ES, Lessell S . Endothelial cell loss in irradiated optic nerves. Ophthalmology 2000; 107: 370–374.
Pe'er J, Stefani FH, Seregard S, Kivela T, Lommatzsch P, Prause JU et al. Cell proliferation activity in posterior uveal melanoma after Ru-106 brachytherapy: an EORTC ocular oncology group study. Br J Ophthalmol 2001; 85: 1208–1212.
Bothman L . Glaucoma following irradiation. Arch Ophthalmol 1940; 23: 1198–1212.
Gragoudas ES, Caroll JM . Multiple choroidal metastasis from bronchial carcinoid treated with photocoagulation and proton beam irradiation. Am J Ophthalmol 1979; 87: 299–304.
Gragoudas ES, Seddon J, Goitein M, Verhey L, Munzenrider J, Urie M et al. Current results of proton beam irradiation of uveal melanomas. Ophthalmology 1985; 92: 284–291.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyd, S., Gittos, A., Richter, M. et al. Proton beam therapy and iris neovascularisation in uveal melanoma. Eye 20, 832–836 (2006). https://doi.org/10.1038/sj.eye.6702072
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.eye.6702072
Keywords
This article is cited by
-
Intravitreal bevacizumab for neovascular glaucoma in uveal melanoma treated by proton beam therapy
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
-
The effects of radiation on angiogenesis
Vascular Cell (2013)