Abstract
Purpose
To evaluate the proliferation rates of five human uveal melanoma (UM) cell lines after treatment with amfenac, a cyclooxygenase (COX)-2 inhibitor, and subsequent radiation exposure.
Methods
Five human UM cell lines (92.1, SP6.5, MKT-BR, OCM-1, and UW-1) and one human fibroblast cell line (BJ) were incubated with amfenac. Treated and non-treated cell lines were then exposed to various doses of γradiation: 0, 2, 4, 6, and 8 Gy. Sulphorhodamine-B assay was used to assess proliferation rates 48 h post-radiation.
Results
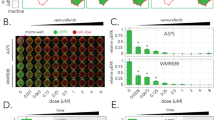
Treatment of UM cell lines with amfenac prior to radiation led to a marked reduction in proliferation rates. This difference was statistically significant in all cell lines at every radiation dose (P<0.005), with the exception of 92.1 at 2 Gy (P=0.157). Fibroblasts treated with amfenac showed significantly higher proliferation rates after 2 and 8 Gy, with no significant differences at 0, 4, and 6 Gy.
Conclusions
The radiosensitivity of UM cell lines was increased by the administration of amfenac, the active metabolite of nepafenac. There appears to be a radioprotective effect of amfenac on human fibroblasts. The topical administration of nepafenac may decrease tumour recurrence and radiation-induced complications while broadening the indications for radiotherapy by treating larger tumours.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM . Epidemiologic aspects of uveal melanoma. Surv Ophthalmol 1988; 32 (4): 239–251.
The Collaborative Ocular Melanoma Study Group. The collaborative ocular melanoma study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma I: characteristics of patients enrolled and not enrolled. COMS report no. 9. Am J Ophthalmol 1998; 125 (6): 767–778.
Bell DJ, Wilson MW . Choroidal melanoma: natural history and management options. Cancer Control 2004; 11 (5): 296–303.
Zhao DY, Shields CL, Shields JA, Gunduz K . Update on the management of posterior uveal melanoma. J Ophthalmic Nurs Technol 1998; 17 (2): 66–71.
Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS . Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM radiation therapy committee task group no. 43. American association of physicists in medicine. Med Phys 1995; 22 (2): 209–234.
Shields CL, Shields JA, Cater J, Gunduz K, Miyamoto C, Micaily B et al. Plaque radiotherapy for uveal melanoma: long-term visual outcome in 1106 consecutive patients. Arch Ophthalmol 2000; 118 (9): 1219–1228.
Puusaari I, Heikkonen J, Kivela T . Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology 2004; 111 (9): 1768–1777.
Smith WL, DeWitt DL, Garavito RM . Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000; 69: 145–182.
Patel MI, Subbaramaiah K, Du B, Chang M, Yang P, Newman RA et al. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res 2005; 11 (5): 1999–2007.
Tang TC, Poon RT, Lau CP, Xie D, Fan ST . Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol 2005; 11 (13): 1896–1902.
Dai Y, Zhang X, Peng Y, Wang Z . The expression of cyclooxygenase-2, VEGF and PGs in CIN and cervical carcinoma. Gynecol Oncol 2005; 97 (1): 96–103.
Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K . Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res 2004; 10 (24): 8465–8471.
Figueiredo A, Caissie L, Callejo SA, McLean IW, Gold P, Burnier Jr MN . Cyclooxygenase-2 expression in uveal melanoma: novel classification of mixed-cell-type tumours. Can J Ophthalmol 2003; 38 (5): 352–356.
Williams CS, Sheng H, Brockman JA, Armandla R, Shao J, Washington MK et al. A cyclooxygenase-2 inhibitor (SC-58125) blocks growth of established human colon cancer xenografts. Neoplasia 2001; 3 (5): 428–436.
Kojima M, Morisaki T, Uchiyama A, Doi F, Mibu R, Katano M et al. Association of enhanced cyclooxygenase-2 expression with possible local immunosuppression in human colorectal carcinomas. Ann Surg Oncol 2001; 8 (5): 458–465.
Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC et al. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res 2005; 11 (4): 1618–1628.
Evans DM, Sloan Stakleff KD . Control of pulmonary metastases of rat mammary cancer by inhibition of uPA and COX-2, singly and in combination. Clin Exp Metastasis 2004; 21 (4): 339–346.
Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342 (26): 1946–1952.
Arun B, Goss P . The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol 2004; 31 (2 Suppl 7): 22–29.
Liao Z, Komaki R, Milas L, Yuan C, Kies M, Chang JY et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable performance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res 2005; 11 (9): 3342–3348.
Liu W, Chen Y, Wang W, Keng P, Finkelstein J, Hu D et al. Combination of radiation and celebrex (celecoxib) reduce mammary and lung tumor growth. Am J Clin Oncol 2003; 26 (4): S103–S109.
Liang L, Hu D, Liu W, Williams JP, Okunieff P, Ding I et al. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol 2003; 26 (4): S114–S121.
Takahashi K, Saishin Y, Mori K, Ando A, Yamamoto S, Oshima Y et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci 2003; 44 (1): 409–415.
Kapin MA, Yanni JM, Brady MX, McDonough TJ, Flanagan JG, Rawji MH et al. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation 2003; 27 (5): 281–291.
Marshall JC, Caissie AL, Callejo SA, Antecka E, Burnier Jr MN . Cell proliferation profile of five human uveal melanoma cell lines of different metastatic potential. Pathobiology 2004; 71 (5): 241–245.
De Waard-Siebinga I, Blom DJ, Griffioen M, Schrier PI, Hoogendoorn E, Beverstock G et al. Establishment and characterization of an uveal-melanoma cell line. Int J Cancer 1995; 62 (2): 155–161.
Diebold Y, Blanco G, Saornil MA, Fernandez N, Lazaro MC . Morphologic and immunocytochemical characterization of four human uveal cell lines (melanoma- and melanocytes-derived). Curr Eye Res 1997; 16 (5): 487–495.
Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM . Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation 2000; 24 (4): 357–370.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990; 82 (13): 1107–1112.
Davis TW, Hunter N, Trifan OC, Milas L, Masferrer JL . COX-2 inhibitors as radiosensitizing agents for cancer therapy. Am J Clin Oncol 2003; 26 (4): S58–S61.
Grimes KR, Warren GW, Fang F, Xu Y, St Clair WH et al. Cyclooxygenase-2 inhibitor, nimesulide, improves radiation treatment against non-small cell lung cancer both in vitro and in vivo. Oncol Rep 2006; 16 (4): 771–776.
Sun Y, Tran BN, Worley LA, Delston RB, Harbour JW . Functional analysis of the p53 pathway in response to ionizing radiation in uveal melanoma. Invest Ophthalmol Vis Sci 2005; 46 (5): 1561–1564.
van den Aardweg GJ, Naus NC, Verhoeven AC, de Klein A, Luyten GP . Cellular radiosensitivity of primary and metastatic human uveal melanoma cell lines. Invest Ophthalmol Vis Sci 2002; 43 (8): 2561–2565.
Furuta Y, Hunter N, Barkley Jr T, Hall E, Milas L . Increase in radioresponse of murine tumors by treatment with indomethacin. Cancer Res 1988; 48 (11): 3008–3013.
Ke TL, Graff G, Spellman JM, Yanni JM . Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 2000; 24 (4): 371–384.
Lane SS . Nepafenac: a unique nonsteroidal prodrug. Int Ophthalmol Clin 2006; 46 (4): 13–20.
Saornil MA, Fisher MR, Campbell RJ, Robertson DM, Earle JD, Eagle Jr RC et al. Histopathologic study of eyes after iodine I 125 episcleral plaque irradiation for uveal melanoma. Arch Ophthalmol 1997; 115 (11): 1395–1400.
Lane SS, Modi SS, Lehmann RP, Holland EJ . Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg 2007; 33 (1): 53–58.
Acknowledgements
Part of this work was presented as a poster at ARVO 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial conflict of interest regarding this article.
Rights and permissions
About this article
Cite this article
Fernandes, B., Marshall, JC., Di Cesare, S. et al. Amfenac increases the radiosensitivity of uveal melanoma cell lines. Eye 22, 701–706 (2008). https://doi.org/10.1038/sj.eye.6703042
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.eye.6703042
Keywords
This article is cited by
-
Management of uveal melanomas: cycloxygenase-2 as a potential molecular target
International Ophthalmology (2012)