Abstract
Although numerous studies have indicated that diapause is heritable and phenotypically plastic, none of them has examined the quantitative genetic basis of this plasticity. In this paper we report such an analysis for egg diapause in the cricket Allonemobius socius, the induction of which appears to be largely determined by the mother. We analysed the quantitative genetic basis of the phenotypically plastic response of female A. socius to age and environmental conditions. We measured the production of diapause eggs on four occasions over a 16-day period, and in two environments; one mimicking an ‘early’ period of the year and another mimicking a ‘late’ period. We analysed genetic variation in phenotypic plasticity using the character-state approach. Diapause proportion was heritable (h2 ranged from 0.17 to 0.49, being larger in the ‘early’ environment), and the genetic correlation between ages in proportion of diapausing eggs was close to 1 but showed a decrease with increased difference between ages. There were significant genetic correlations between environments for all ages. Because of the reduction in genetic correlation as the difference in ages increases, selection will be more effective at changing the overall shape of the reaction norm than causing local changes. Furthermore, the high genetic correlations may constrain the evolution of the reaction norm. When the two environments are converted into the estimated days in the year the two reaction norms form approximately a single curve as predicted from previous theoretical analysis of the optimal reaction norm.
Similar content being viewed by others
Introduction
In a heterogeneous environment there may be selection for the ability to respond to cues that give information about the present or future state of the environment. Such phenotypic plasticity results in a mapping between phenotype and environment that is known as the reaction norm (for a review of the concept see Schlichting & Pigliucci, 1998). Reaction norms are found in a wide range of characters, particularly life-history traits (Travis, 1994). It is a general finding that phenotypic plasticity is genetically variable (e.g. see table 6.1 in Roff, 1997) and hence selection should generally be capable of moulding the reaction norm to its optimum (De Jong, 1990; but see Roff, 1994 and De Jong, 1999 for cases in which evolution will be constrained).
Diapause induction is an ideal model for the genetic analysis of the evolution of reaction norms, because the fitness advantages of entering diapause at a particular time can be readily modelled using life-history data and historical data such as temperature records (Bradford & Roff, 1997). There is considerable evidence that diapause induction is heritable and is phenotypically plastic (Tauber et al., 1986), although to our knowledge there are no studies showing that this phenotypic plasticity is under polygenic control. The purpose of the present study was to analyse the genetic variability of phenotypic plasticity for diapause induction in the cricket Allonemobius socius.
Sib analysis and common garden experiments have shown genetic differentiation among populations for diapause induction in the eggs of A. socius (Mousseau & Roff, 1989; Mousseau, 1991; Bradford & Roff, 1995), and that diapause is sensitive to temperature, photoperiod (Bradford & Roff, 1995) and maternal age (Mousseau, 1991; Bradford & Roff, 1993). Female A. socius from a bivoltine population lay a larger proportion of nondiapausing eggs when reared under conditions mimicking early summer than when reared under late summer conditions (Bradford & Roff, 1995), whereas the proportion of diapausing eggs increases with the age of the female (Bradford & Roff, 1993). The specific objective of the present study was to determine the quantitative genetic basis of these two types of phenotypic plasticity (between environment, between ages).
Materials and methods
Species description
Allonemobius socius is a small (≈0.07 g) common ground cricket of the subfamily Nemobiinae. It is found in wet grasslands in the south-eastern United States from Florida to New Jersey (Howard & Furth, 1986). In the northern part of its range A. socius is univoltine, becoming bivoltine in Virginia and possibly multivoltine in Florida (Howard & Furth, 1986). The transition from a univoltine to bivoltine phenology occurs between latitudes 34–37°N, in which region voltinism is primarily a conditional strategy and not a simple genetic polymorphism (Bradford & Roff, 1995). In the transition area, overwintering eggs hatch in May and first-generation adults appear in July and early August. Females of the first generation produce mixtures of nondiapausing and diapausing eggs (Mousseau, 1991). Eggs laid in August or later typically diapause: thus second-generation females produce only diapausing eggs.
Individuals used in the present experiment were from the third generation of a stock descended from approximately 100 adults collected in July (1988) from Danville, Virginia (36°40′N). Husbandry methods were as described in Bradford & Roff (1993).
Experimental design
A split family full-sib design was employed to estimate genetic parameters. Although such estimates are potentially biased by nonadditive genetic effects, space limitations and difficulties with mating prevented us from using the preferred half-sib design.
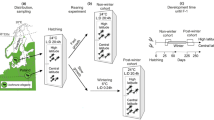
The parental generation was reared under short photoperiods so that all eggs from the pair-mating were in diapause. The eggs were kept at 4°C for 3 months, and were then warmed to 28°C to initiate hatching. This synchronized development such that the hatching of offspring after diapause was concentrated over a 2- to 3-day period. Upon hatching, nymphs from each family were placed in one of two environments designed to span the range of conditions experienced by the first generation in this population. The ‘early’ environment consisted of a photoperiod of 15.5:8.5 h light:dark (L:D) for nymphs and 15:9 h L:D for adults, corresponding approximately to photoperiods (including civil twilight) between Julian days 195 and 210 at the collection site of the experimental population. In the ‘late’ environment, conditions were set at 15:9 h L:D for the nymphs and 14.5:9.5 h L:D for the adults, simulating Julian days 210 and 225. In all environments a 31:19°C thermoperiod was used on a 12-h cycle, with the increase in temperature set at 1.5 h before lights on. These are average midsummer temperatures for the collection site. There were 56 families that had sufficient (>20) eggs to make up two replicates per environment and nine families that yielded enough eggs for only two replicates in a single environment; the latter were all placed in the early environment.
Mousseau & Roff (1989) estimated the heritability of diapause propensity, assuming that it was a trait of the offspring. Further research suggested that it might more properly be considered a maternal trait (Tanaka, 1986; Mousseau, 1991; Bradford & Roff, 1995) and thus in the present experiment we used the proportion of eggs diapausing as a trait of the female, not the egg itself. For each environment 6–8 females were chosen haphazardly from each family and mated and allowed to reproduce for the estimation of diapause proportion. To ensure successful mating each female was provided with two randomly chosen males, either from the pool of males emerging from the experiment or from a separate rearing of the surplus nymphs. We collected four batches of eggs at 4-day intervals from each female, beginning on the ninth day after the final moult (i.e. covering days 9–12, 13–16, 17–20, 21–24). Eggs were incubated in the same environment as the mothers for 14–18 days, at which point diapause, direct-developing and infertile eggs were scored. Infertile eggs were not used in the calculation of diapause proportion and batches of fewer than four eggs were excluded from the analysis. The total number of egg batches (where one egg batch corresponds to the output of a single female) obtained for each period and in each environment were: ‘early’ environment, 392, 433, 401, 365; ‘late’ environment, 345, 370, 372, 338.
Statistical methods: initial test
We first tested for variation in proportion diapause between environments, among ages and families using both univariate and multivariate repeated measures ANOVA. For these analyses we used only families that were represented in both environments. Additionally two families had to be dropped, as their inclusion, for reasons we could not ascertain, generated a matrix singularity. Following the finding that there was significant variation attributable to both age and environment we estimated heritabilities and genetic correlations (rA) separately for each environment/age combination as described below.
Statistical methods: female age and proportion of diapause eggs
We calculated the heritability of the proportion of diapause eggs at each age (designated Pi, where i=1, 2, 3, 4, for the first, second, third and fourth collection) and the genetic correlation between them (use of the arcsine square root transformation did not change the results and we report here the results on the raw scores). Heritabilities and their associated standard errors were estimated using a delete-one (=family) jackknife with the pseudovalues estimated from a nested (nymphal rearing cage nested within family) one-way ANOVA as described in Simons & Roff (1994); as there was no significant effect attributable to rearing cage for the nymphs the results presented here are for the combined data (no nesting).
The distribution of diapause proportions among females was skewed, with approximately 50% of females in any sample producing only diapause eggs, which potentially biased the ANOVA estimates. Therefore, we confirmed the ANOVA results with the randomization method described in Roff & Bradford (1996).
Genetic correlations between ages within the same environment were estimated using ANOVA combined with the delete-one (family) jackknife as described in Roff & Preziosi (1994). Only families for which data were available for both environments were used in this analysis (this is necessary for the jackknife procedure used). This reduced the number of families to 56.
Statistical methods: estimation of the genetic correlations between environments
We used the mixed-model ANOVA method with families as a random effect and environments and age as fixed effects to test for a positive genetic correlation (Fry, 1992). Because the data were not fully balanced, this test should be considered approximate. Genetic correlations were determined by estimating separately by REML (using the varcomp procedure in SPLUS) the covariance and the two variances (Fry, 1992; correlations obtained from family mean values were used to confirm that the correlations were positive). Standard errors were estimated using the jackknife (Roff & Simons, 1997; Windig, 1997). As an alternative to the mixed-model ANOVA we used a one-sample t-test [t=(rA − x)/SE], where rA and SE were estimated using the jackknife and x was zero or one (Knapp et al., 1989).
There are two ‘types’ of genetic correlations between environments: that between the same trait (e.g. P1) and that between two different traits (e.g. P1 and P2). In the first case there is only one way to obtain the jackknifed estimate. In the second case there are two ways, e.g. P1 in the ‘early’ environment vs. P2 in the ‘late’ environment, or P2 in the ‘early’ environment vs. P1 in the ‘late’ environment. Thus two sets of pseudovalues from the jackknife procedure are estimated; under the null hypothesis that the two sets of data do not differ the difference between the mean pseudovalues will be zero. This was tested by means of a two-sample t-test. If the two samples were not found to differ, the genetic correlation was estimated using the combined sample of jackknife pseudovalues.
Results
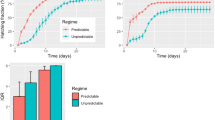
The proportion of eggs laid in the ‘late’ environment that were in diapause was approximately 0.1 greater than in the ‘early’ environment (Fig. 1). In both environments there was a trend towards a decline in proportion of diapause eggs at age 16. The univariate and multivariate repeated measures ANOVAs gave the same results as did the probabilities estimated from the F, Greenhouse–Geiser and Huynh–Feldt statistics: we present only the results for the univariate test using the F-values (Table 1). There were highly significant effects of family, environment and age but the only significant interaction was between age and family. These results suggest that the shapes of the reaction norms were the same in both environments, which is apparent from the plots of the means (Fig. 1), but that there was also significant genetic variation averaged over environments in age-specific tendency to produce diapausing eggs.
Heritabilities
Both the estimated standard errors and randomization test indicated that there was significant genetic variation for diapause proportion at all ages (Table 2). The heritability estimates for the proportion of diapause eggs at each age did not vary substantially with age but were consistently higher in the ‘early’ environment (Table 2). We tested for a difference between heritabilities in the different environments by means of a paired t-test (i.e. the signed difference between Pi in the two environments). The mean difference of 0.20 was highly significant (t3=5.43, P=0.012). The increased heritability in the ‘early’ environment was caused primarily by an increase in the additive genetic variance (mean VA in ‘early’ environment=0.07 vs. 0.03 in the ‘late’ environment), with the environmental variance remaining more or less constant (mean VE in both environments=0.09: Table 2).
Genetic correlations between ages within each environment
All phenotypic and genetic correlations between the proportion of diapausing eggs produced at different ages were significantly different from zero (mean − 2 SE > 0 in all cases; note that because of sample size this test is equivalent to a test based on the estimated t-value, Table 3). The phenotypic correlation was approximately 0.60 and was significantly less than 1 (mean + 2 SE <1 in all cases), whereas the genetic correlation was generally very close to 1 and in no case was it significantly different from 1 (mean + 2 SE > 1 in all cases). In all cases the genetic correlation was larger than the phenotypic correlation (Table 3); because these two statistics are not independent there is no appropriate statistical test for the observed difference, but the consistency of the result does suggest that the difference is real. The difference between the genetic and phenotypic correlations results from difference in both the covariances (Cov) (‘early’ environment: mean CovA=0.070, mean CovP=0.102, mean ratio of CovA/ CovP=0.69; ‘late’ environment: mean CovA= 0.024, mean CovP=0.073, mean ratio of CovA/ CovP=0.33) and the denominators (Denom) (‘early’ environment: mean DenomA=0.072, mean DenomP= 0.152, mean ratio of DenomA/DenomP=0.46; ‘late’ environment: mean DenomA=0.029, mean DenomP= 0.115, mean ratio of DenomA/DenomP=0.25).
The ‘within-environmental’ genetic and phenotypic correlations were larger, on average, in the ‘early’ environment (‘early’ environment: mean rP=0.66, mean rA=0.97; ‘late’ environment: mean rP=0.63, mean rA=0.84). As an approximate test of whether the ‘within-environmental’ correlations differed we used a paired t-test. The genetic correlations did not differ (t5=1.60, P=0.170), whereas the phenotypic correlations were marginally nonsignificant (t5=2.33, P=0.067).
In general, we might expect that the more distant two traits are then the lower will be the genetic correlation between them: thus the genetic correlation between two ages is predicted to decline as the difference in ages increases. For both the phenotypic and genetic correlations there was a statistically significant decline in the correlation as the difference between ages increased. For each type of correlation there are six comparisons and in all comparisons the correlation declined; from the binomial test the probability of all six comparisons going in the predicted direction is 0.015 (one-tailed probability).
Genetic correlations between environments
The two-sample t-tests indicated no difference between the two possible estimates of rA for different traits (P > 0.2 in all six possible combinations); we therefore combined the data. With one exception (P4) the genetic correlations were all highly significantly different from zero (P < 0.001, one-tailed t-tests: Table 4). As with the genetic correlations within environments, there was a trend for the correlation to decrease as the difference in ages increased (five decreases out of six possible, P=0.1094, binomial one-tailed test). The overall pattern (nine of 10 estimates <1) suggests that rA was less than one. This is also suggested by the almost significant family × environment interaction in the repeated measures ANOVA (Table 1). The grand mean of all estimates equalled 0.67 (SE=0.065, n=10) which was significantly different from unity (t9=5.03, P=0.001).
Discussion
By using a growth chamber programmed to produce a continuously changing environment that mimicked the area from where the present stock of A. socius was collected, Bradford & Roff (1993) found that females laid approximately 30% of diapausing eggs on day 210 of the year (‘early’ environment) and 78% of diapausing eggs on day 225 (‘late’ environment). The latter proportion is approximately what we obtained in the ‘late’ environment but the former is less than one-half that observed in the ‘early’ environment (Fig. 1). This discrepancy suggests that a continuously changing photoperiod and/or temperature is important in inducing diapause, as has been found in the butterfly, Polygonia c-album (Nylin, 1989). Nevertheless, there was a significant difference in the diapause response that was qualitatively consistent with the expected pattern of increased proportion diapause in the ‘late’ environment. Thus under the two experimental regimes females showed phenotypic plasticity for diapause induction.
The proportion of diapause eggs increased overall with female age (Fig. 1; Mousseau, 1991; Bradford & Roff, 1993), indicating that diapause was at least partially determined by the mother. The slight decrease in proportion of diapause eggs produced during the second period (age 13–16 days) may be a consequence of the sudden shift between photoperiods at the time of eclosion into the adult. However, given that the photoperiodic shift should have indicated less time remaining before the end of the growing season, we would have expected an increase rather than a decrease.
In this paper we have assumed that diapause was a maternal trait that in a given environment can be characterized by the proportion of diapause eggs produced at different ages (Pi). The heritabilities of Pi were larger in the ‘early’ than the ‘late’ environment but significant in both (Table 2). The genetic correlations between days were all very close to one but did show a significant decline as the differences in ages increased (Table 3). Such declines have been observed between morphological traits at different ages in mammals (Atchley, 1984) and fecundity in Drosophila melanogaster (Engstrom et al., 1992). If the genetic correlation between ages were actually one, then selection could not change the shape of the reaction norm. The slight reduction below one means that the reaction norm can evolve. Furthermore, the decline with the age difference means that selection can more readily change the overall shape of the reaction norm than it can make local variation in shape.
Mousseau & Roff (1989) estimated the heritability of diapause on the assumption that it was a trait of the offspring. They obtained an average estimate of 0.74, which is higher than obtained in the present analysis. If, as appears to be the case, the assumption that diapause induction is an offspring trait is incorrect then their previous estimates of heritability are inflated, and are equal to twice the repeatability of the trait (i.e. twice the intraclass correlation; Lessells & Boag, 1987). The population (Richmond) studied in Mousseau & Roff (1989) that was geographically the closest to the population in the present study gave a repeatability of 0.45. The conditions under which the Richmond population was raised (30°C, 14:10 h L:D) most closely match the ‘early’ environment of the present experiment under which conditions the heritability of diapause proportion varied from 0.40 to 0.49, with a mean of 0.45 (Table 2), which is the same as the repeatability from the Mousseau & Roff study. This suggests that the correct heritability of diapause for the populations analysed in Mousseau & Roff (1989) can be obtained by halving their reported values. Using this correction, the heritabilities ranged from 0.15 to 0.55, with a mean of 0.37, which is similar to that obtained in the present analysis. Similar estimates for the heritability of diapause have been obtained in a lepidopteran, Choristoneura rosaceanna (h2=0.38, SE=0.10; Carrière, 1994) and a copepod, Diaptomus sanguineus (h2=0.60, SE=0.35; Hairston & Dillon, 1990). Significant additive genetic variance has also been found in two other aspects of diapause; degree days from chilling to adult eclosion in Hyphantria cunea (h2=0.60, SE=0.22; Morris, 1971), and the critical photoperiod for the induction of diapause in Wyeomyia smithii (h2=0.70, SE=0.16; Bradshaw & Holzapfel, 1990).
In each year there is a particular day on which a female should switch from laying nondiapausing eggs to diapausing eggs; in some populations of copepods this switch is determined ultimately by fish predation (Hairston & Dillon, 1990), whereas in A. socius the important selective factor appears to be the probability that there remains sufficient time to complete development and produce another batch of eggs, which is the only stage that can diapause (Bradford & Roff, 1997). Interannual variation in the occurrence of fish or length of time suitable for development will favour a flexible timing for the switch between diapause and nondiapausing eggs. If a female A. socius can assess the day of the year but not the remaining length of the growing season, selection will favour a reaction norm in which mixed batches of diapause and nondiapause eggs are produced on some days. Using historical temperature data Bradford & Roff (1997) determined this reaction norm for A. socius in the region from which the experimental animals originated. The transition from laying only nondiapause eggs to laying only diapause eggs was predicted to be very narrow, covering approximately a 12-day period. This rapid change is consistent with the observed coefficient of variation in season length of only 10–15% (Bradford & Roff, 1997). These results are also consistent with other studies of risk-spreading in insects (Hopper, 1999).
According to the assumptions of the model of Bradford & Roff (1997) the reaction norms obtained in the two experimental environments should be the ‘lower’ and ‘upper’ portions of a single reaction norm. Alternatively, the reaction norm might consist of a more complex function that includes both age and environment, as suggested by the nearly significant family × environment interaction (Table 1). However, the following evidence suggests that the major factor is relative age. The early environment covers approximately the period encountered by the adult during the monitored egg-laying period from day 222 to 234 whereas the late environment covers days 237 to 249 and therefore the curve for the late environment should be a continuation of that found in the early environment. A right translational shift of the curve from the late environment does produce a more or less single reaction norm (Fig. 2). Under the hypothesis of a single reaction norm the genetic correlation across environments corresponds to the genetic correlation between days that are separated by the appropriate number of days. Thus, for example, the genetic correlation between P1 in the ‘early’ environment and P1 in the ‘late’ environment should correspond to the genetic correlation between any two ages separated by approximately 15 days (i.e. days 222 and 237 in (Fig. 2). Using this criterion we plotted the genetic correlation as a function of the expected or ‘perceived’ separation in days and, as predicted, found a highly significant negative regression (r=−0.70, n=21, P=0.0005).
Diapause curves as shown in Fig. 1 but plotted according to the approximate day of the year.
Genetic variation in the reaction norm will allow selection to modify the proportion of diapause eggs produced as conditions change either over time or over space. This prediction has been verified by a common garden experiment using three geographical stocks of A. socius raised in three environments mimicking the locations from where the stocks were collected (Bradford & Roff, 1995). Diapause propensity varied with environment and stock, indicating genetic variation among populations and a response to selection, presumably caused in part by the different season lengths at the different locations. In an environment in which the season length is typically too short for more than a single generation, selection will favour females in which there is an early age-specific switch to diapausing eggs and the reaction norm is largely unaffected by external cues. Similarly, at a location at which a bivoltine life history is favoured, in most years selection will favour females with reaction norms that show a strong interaction with external cues (e.g. photoperiod and/or temperature) such that first generation females show a very late age-specific switch to diapause whereas females from the second generation show an early age-specific switch to diapausing eggs. The common garden experiments described above support these predictions: a univoltine population raised in its natal environment produced only diapause eggs, whereas females from a bivoltine population and females from a mixed (transitional) population produced some direct developing eggs early in their reproductive schedule (Bradford & Roff, 1995). Similarly, females from the bivoltine population when raised in their natal environment (conditions mimicking early summer) produced only direct-developing eggs, whereas females from the univoltine and mixed populations produced some diapausing eggs. In any particular environment the proportion of diapausing eggs increased with the latitude of origin of the females.
Diapause is a central feature of the life histories of many invertebrates, as is its equivalent, dormancy, in many plant species. Whereas there is evidence that diapause induction is, in general heritable, the present study is the first to estimate the genetic parameters of the reaction norm. The high genetic correlations between ages and between environments indicate that the evolution of the reaction norm can be constrained and the evolutionary trajectory towards at least some reaction norms may be very long.
References
Atchley, W. R. (1984). Ontogeny, timing of development, and genetic variance–covariance structure. Am Nat, 123: 519–540.
Bradford, M. J. and Roff, D. A. (1993). Bet hedging and the diapause strategies of the cricket Allonemobius fasciatus. Ecology, 74: 1129–1135.
Bradford, M. J. and Roff, D. A. (1995). Genetic and phenotypic sources of life history variation along a cline in voltinism in the cricket Allonemobius socius. Oecologia, 103: 319–326.
Bradford, M. J. and Roff, D. A. (1997). An empirical model of diapause strategies in the cricket Allonemobius socius. Ecology, 78: 442–451.
Bradshaw, W. E. and Holzapfel, C. M. (1990). Evolution of phenology and demography in the pitcher plant mosquito, Wyeomyia smithii. In: Gilbert, F. (ed.) Insect Life Cycles: Genetics, Evolution, and Co-ordination, pp. 47–67. Springer, London.
Carrière, Y. (1994). Evolution of phenotypic variance: non-Mendelian parental influences on phenotypic and genetic components of life-history traits in a generalist herbivore. Heredity, 72: 420–430.
De Jong, G. (1990). Quantitative genetics of reaction norms. J Evol Biol, 3: 447–468.
De Jong, G. (1999). Unpredictable selection in a structured population leads to local genetic differentiation in evolved reaction norms. J Evol Biol, 12: 839–851.
Engstrom, G., Liljedahl, L. -E. and Bjorklund, T. (1992). Expression of genetic and environmental variation during ageing. 2. Selection for increased lifespan in Drosophila melanogaster. Theor Appl Genet, 85: 26–32.
Fry, J. D. (1992). The mixed-model analysis of variance applied to quantitative genetics: biological meaning of the parameters. Evolution, 46: 540–550.
Hairston, N. G. Jr and Dillon, T. A. (1990). Fluctuating selection and response in a population of freshwater copepods. Evolution, 44: 1796–1805.
Hopper, K. R. (1999). Risk spreading and bet-hedging in insect population biology. Ann Rev Ent, 44: 535–560.
Howard, D. J. and Furth, D. G. (1986). Review of the Allonemobius fasciatus (Orthoptera: Gryllidae) complex with the description of two new species separated by electrophoresis, songs and morphometrics. Ann Entomol Soc Am, 79: 472–481.
Knapp, S. J. Jr, Bridges, W. C. and Yang, M. (1989). Nonparametric confidence estimators for heritability and expected selection response. Genetics, 121: 891–898.
Lessells, C. M. and Boag, P. T. (1987). Unrepeatable repeatabilities: a common mistake. Auk, 104: 116–121.
Morris, R. F. (1971). Observed and simulated changes in genetic quality in natural populations of Hyphantria cunea. Can Ent, 103: 893–906.
Mousseau, T. A. (1991). Geographic variation in maternal-age effects on diapause in a cricket. Evolution, 45: 1053–1059.
Mousseau, T. A. and Roff, D. A. (1989). Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution, 43: 1483–1496.
Nylin, S. (1989). Effects of changing photoperiods in the life cycle regulation of the comma butterfly, Polygonia c-album (Nymphalidae). Ecol Entomol, 14: 209–218.
Roff, D. A. (1994). The evolution of dimorphic traits: predicting the genetic correlation between environments. Genetics, 136: 395–401.
Roff, D. A. (1997). Evolutionary Quantitative Genetics. Chapman & Hall, New York.
Roff, D. A. and Bradford, M. J. (1996). Quantitative genetics of the trade-off between fecundity and wing dimorphism in the cricket Allonemobius socius. Heredity, 76: 178–185.
Roff, D. A. and Preziosi, R. (1994). The estimation of the genetic correlation: the use of the jackknife. Heredity, 73: 544–548.
Roff, D. A. and Simons, A. M. (1997). The quantitative genetics of wing dimorphism under laboratory and ‘field’ conditions in the cricket Gryllus pennsylvanicus. Heredity, 78: 235–240.
Schlichting, C. D. and Pigliucci, M. (1998). Phenotypic Evolution—a Reaction Norm Perspective. Sinauer Associates, Sunderland, MA.
Simons, A. M. and Roff, D. A. (1994). The effect of environmental variability on the heritabilities of traits of a field cricket. Evolution, 48: 1637–1649.
Tanaka, S. (1986). Developmental characteristics of two closely related species of Allonemobius and their hybrids. Oecologia, 69: 388–394.
Tauber, M. J., Tauber, C. A. and Masaki, S. (1986). Seasonal Adaptations of Insects. Oxford University Press, New York.
Travis, J. (1994). Ecological genetics of life-history traits: variation and its evolutionary significance. In: Real, L. A. (ed.) Ecological Genetics, pp. 171–204. Princeton University Press, Princeton, NJ.
Windig, J. J. (1997). The calculation and significance testing of genetic correlations across environments. J Evol Biol, 10: 853–874.
Acknowledgements
We gratefully thank J. Windig, P. Crnokrak and G. Stirling for their helpful comments. This research was supported by a grant to D.A.R. from the Natural Sciences and Engineering Council of Canada, and by grants from NSERC and Max Bell postgraduate scholarships to M.J.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roff, D., Bradford, M. A quantitative genetic analysis of phenotypic plasticity of diapause induction in the cricket Allonemobius socius. Heredity 84, 193–200 (2000). https://doi.org/10.1046/j.1365-2540.2000.00650.x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00650.x