Abstract
The quantitative genetic properties are derived for the bulk F2 originating from random fertilization (RF) amongst hybrid (F1) individuals. Only its mean appears to have been derived previously, and that definition is confirmed (by another method). New general equations are found also for all genotype frequencies, allele frequencies, inbreeding coefficient, the genotypic, additive-genetic and dominance variances, and broad-sense and narrow-sense heritabilities. The assumption that such an F2 is a classical RF population is shown to be correct. Indeed, the allogamous F2 is a natural origin for the RF population. The relationships are given between precedent RF populations (parents) and subsequent RF populations following hybridization (allogamous F2). The allogamous F2 is generally inbred with respect to its parental F1, the degree depending on the hybrid’s parents’ allele frequencies. At the same time, it is outbred with respect to those original parents, and not inbred at all with respect to the equivalent RF population. The genotypic variance is generally more than in the F1, and likewise for heritabilities. These findings make it possible to evaluate the genetic advance from selection and hybridization. The results depend on the allele frequencies of the original parents and the degree of overdominance, but generally, selection is more advantageous than hybrid vigour.
Similar content being viewed by others
Introduction
Many episodes in evolution and plant breeding are initiated by hybridizing between parent populations or individuals. Meiosis and syngamy within the hybrid (F1) lead to a population with a different frequency structure (the F2), i.e. the F1 is in disequilibrium and has an ephemeral population structure. The quantitative genetic properties of the F1 population have been examined (Gordon, 1999), but how do the properties of the emergent F2 relate to these? Intuitively, because of meiosis, we would expect more variance: but we need to explore the quantitative relationships of this matter. Intuitively, also, we would expect to have to account for effects of inbreeding because of shared ancestral origins (F1 individuals) amongst members of the F2 population. This needs to be reconciled with the assumption that an allogamous F2 can be considered as a single panmictic random fertilizing gamodeme with zero inbreeding. It appears that these, and related, matters have not been explored rigorously nor quantitatively.
These omissions became strongly apparent when the author sought to estimate the genetic advance arising from selection from an F2. This is a fundamental phase in important protocols of plant breeding, such as Line Breeding and Pedigree Breeding (Allard, 1960; Poehlmann, 1979; Moore and Janik, 1983). Such selection from the F2 is essential also to the ongoing contribution of a hybrid in natural selection. In order to evaluate genetic advance from such selection, we need more knowledge on the properties of the F2.
The present paper examines the quantitative genetic properties of the bulk F2 population generated by random fertilization amongst F1 individuals. Some earlier focus has examined the mean of such an F2 (Falconer, 1981), in order to demonstrate the drop in hybrid vigour compared with the F1. Here, we derive as well the allogamous F2 genotypic variance (σ2G), genic variance (σ2A), dominance variance (σ2D), broad-sense and narrow-sense heritabilities (h2B and h2N, respectively), genotypic and allelic frequencies, and levels of inbreeding. Following this, we will be able to estimate selection genetic advance from the F2 and we will examine the relationship of the allogamous F2 to random fertilizing (RF) populations.
General method
A population consists of genotypes arising from alleles (or allele Expectations) A1 and A2 present with frequencies p and q respectively, with no omissions (i.e. p + q=1). Its individual phenotypic Expectations are its genotype effects defined as deviates from the homozygote midpoint, namely g′=[a, d, −a] for {A1A1, A1A2, A2A2}, respectively. This is the familiar single-factor gene model used to define classical RF populations (Falconer, 1981). It has been used also to define hybrids (Gordon, 1999), where further discussion is presented on its utility. In examples given in this paper, we will use a=10, and d=7.5 (partial dominance). The population properties also depend on the genotype frequencies; it is one of the tasks of this paper to discover these for the allogamous F2.
The original parent populations set the parameters not only for the hybrid (F1), but also for this F2. The focal parent (P1) is a population of individuals, with allele frequencies p1 and q1. As p1 → 1 (or 0), we are dealing with a pure-line population (or even a homozygous individual); but a focal-parent population may have any p1 within the range 0 ≤ p1 ≤ 1. The other parent (P2) has frequencies defined as offsets from the P1 frequencies, following Falconer (1981). This leads to p2=p1 − y, and q2=q1 + y, where y=p1 − p2. These are the same definitions as used for hybrids (Falconer, 1981; Gordon, 1999).
The RF population analogous to the allogamous F2 is of special relevance, and is based on and (Gordon, 1999). It has the classical properties (Falconer, 1981):

A major task of this paper is to redefine these in terms of the F2 ‘offset’ parameters, and to compare the two sets of results.
All other Methods form an integral part of the derivations which constitute the Results of this paper, and will be presented there.
Results
Genotype frequencies
Allogamous F2
We adopt a fundamental means of deriving the genotype frequencies: we use the F1 genotype frequencies (Gordon, 1999) as parental frequencies, and the cross-products of these form crossing (mating) frequencies. Next, we note the segregation ratios of each family of cross which, when multiplied by the crossing frequencies, gives the family frequencies of segregating progeny genotypes. When these terms are accumulated across all crossing families, we obtain the allogamous F2 genotype frequencies. The F1 parent frequencies and the crossing frequencies are presented in Table 1.
Gathering the outcomes for A1A1 progenies leads to:

Similarly, for A1A2 progenies:

And lastly, for A2A2 progenies:

We define the row-vector containing these results as f′.
These results reveal that for any allogamous F2 from crosses with complementary opposite parents (i.e. p2=1 − p1), the heterozygote frequency is constant (f12=0.5) across all values of p1. Whenever the other parent has a fixed p2, the general levels of heterozygosity are often lower than for the complementary opposites cases. The heterozygosity of any F2 is generally less than that in its corresponding F1; but the two are equal when p1=p2.
Equivalent RF and panmictic
Here, we use the classical RF genotype frequencies, and substitute (p1 − ½y) for and (q1 + ½y) for (Gordon, 1999). Thus:

We notice that these are the same results as obtained for the allogamous F2, proving that the genotype frequencies at least are the same for the two situations. Note that these two results were derived in different but relevant ways, and obtaining the same outcome establishes their equivalence.
Allele frequencies
We need to check the allele frequencies of the F2 using the genotype frequencies f′. This can be done as weighted means using the allele structures of genotypes as weights. Thus, for the A1 allele, the weights are w′1=[1, 1/2, 0] for {A1A1, A1A2, A2A2}, respectively; and for the A2 allele, the respective weights are w′2=[0, 1/2, 1]. The allele frequencies are then found as follows:

and

Note that these are the same as the F1 frequencies (Gordon, 1999), which we would expect. (The RF frequencies were defined to these values at the outset).
Inbreeding coefficient
The natural inbreeding reference population for the F2 is the F1, as the former is sexually derived from the latter. The F2 bulk population is a mixture of many different full-sib lines and half-sib lines, with varying degrees of coancestry amongst them. The simplest approach to defining inbreeding in this bulk mixture is the net drop in heterozygosity of the F2 relative to the F1. This approach precludes any need to unravel the breeding system mixture. Furthermore, it automatically reflects the dynamics of the bulk which depend on the parental inputs (p1). Therefore, the inbreeding coefficient is defined as:

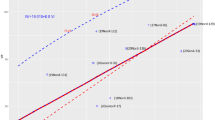
where f12 of the F2 is defined in this paper, and f12 of the F1 is defined in Gordon (1999). The degree of inbreeding in the allogamous F2, for all possible focus-parent inputs (p1), is visualized in Fig. 1, where the other-parent is complementary opposite or fixed p2=0.01, 0.5 or 0.8 as examples.
We observe that the level of inbreeding depends on the relatives-mixture present in the F1 bulk, which changes as does p1. Where p1 → 0 (or 1), for complementary opposite crosses, the hybrid is virtually entirely heterozygous, and the F2 is equivalent to selfing a heterozygote; hence the inbreeding is φ → ½. Again, for this same crossing system, when p1 → p2 → ½, the ‘hybrid’ is virtually a RF population, and the F2 is equivalent to a second-generation RF in equilibrium with the ‘hybrid’. Therefore, there is no change in heterozygosity, and φ → 0.
Now, we will compare this F2 heterozygosity against that of the equivalent RF, as an alternative reference. This is achieved simply by replacing f12 of the F1 with f12 of the RF in the equation for φ. We recall that we have shown that both the allogamous F2 and the equivalent RF (based on ) have the same level of heterozygosity. Therefore, the inbreeding of the allogamous F2 is zero when referenced against the equivalent RF population. This conforms with widespread notions already extant. But it is of doubtful relevance, because the natural reference population is the parental origin, viz. the F1. The whole issue becomes more intriguing still if we use the mean heterozygote frequency of the two parents as the reference basis. We then find that nearly all allogamous F2 are outbred (negative φ) with respect to the parents, except at p1 → ½. This dependence of the value of inbreeding on an appropriate reference base was noted by its pioneer, Sewall Wright (Wright, 1921).
Population mean
Allogamous F2
The F2 mean (F¯2) is , where μ is the attribute background mean, and (=γ also) is the gene-model mean. The latter is obtained as follows:

The α1 is the usual average allele substitution effect for the focal parent population (e.g. Falconer, 1981), and equals a + (q1 − p1)d. This is the same result as presented by Falconer (1981) using another method of derivation, and the −½y2d represents the drop in hybrid vigour, which was his focus. Several examples of this mean are shown in Fig. 2, all for partial dominance (a=10, d=7.5, μ=10). Figure. 2 shows both complementary opposite parents and fixed other-parent p2=0.5, and compares both the F2 and F1 means. The loss of vigour is obvious, especially for p1 → 0 (or 1). Two other F2-mean examples are shown to illustrate extremes, namely fixed p2=0.01 and fixed p2=0.8.
It is interesting to note the patterns of increase in the mean with increasing overdominance. The effect of parental complementarity (i.e. = ½ always) leads to the mean being constant across all p1, but rising in steps with increasing overdominance. The patterns with other forms of parental relationship (with respect to p1 and p2) are more varied, but there is still a general increase in the mean with increasing overdominance.
Equivalent RF and panmictic
We use at once the classical RF definition of the gene-model mean (see General Method), and substitute into it the values for in offset form. Thus:

Through this appropriate independent derivation, we find that the RF mean is the same as the F2 mean. We now have proved that in two properties the allogamous F2 and the RF population are identical, viz. genotype frequencies and gene-model mean.
Genotypic variance
Allogamous F2
The allogamous F2 genotypic variance has the Expectation

where the right-hand terms are an unadjusted sum-of-squares (USS) and a correction factor (CF), respectively. Using the previously defined f′ and g′ vectors and the scalar γ, and after subtracting the CF from the USS followed by gathering and factoring several terms Footnote 1, the SS (i.e. σ2G the genotypic variance Footnote 2), becomes:


Now, recalling the classical definitions of σ2A, and of σ2D (Falconer, 1981), and of cov(ad) and σ2d, (Gordon, 1999), this whole expression resolves into:

The previous equation (eqn 2) is simpler, but this one provides some link with the traditional nomenclature. However, there is some danger in it, for these components are not the genic and dominance variances of the F2 σ2G. We will explore that issue in due course.
The variance can be visualized for our partial dominance and complementary opposites and fixed other-parent p2=½ crosses in Fig. 3. In each case, it is compared also with the σ2G of its F1.
Notice from Fig. 3 that this F2 variance is generally greater than that of its F1, except for the region p1 → ½ where they are virtually equal. This was one of the questions we posed at the beginning. For the complementary opposites cross, the F2 variance greatly exceeds that of its F1 as p1 → 0 (or 1). Another conspicuous result for the complementary opposites is that the variance is constant across all p1; this results from the facts that pF1 is always ½, and F2 genotype frequencies are constant for such crosses. The results for the fixed p2=½ crosses are conspicuously different. These general differences between the F2 and F1 variances have interesting consequences regarding ongoing selection efficiency, and the balances between selection and hybrid vigour, which we will discuss later.
Equivalent RF and panmictic
As before, we establish an independent derivation by using the classical RF approach, and substitute into it the offset structures. Therefore, for the RF base σ2G=σ2A+σ2D, where σ2A is the genic variance and σ2D is the dominance variance. We define each of these separately, and accumulate them at the end.
(i) Genic variance

Substituting previous definitions of σ2A, α, σ2d, cov(a,d) into this, it becomes:

It is convenient subsequently to leave the several σ2d terms separated rather than gathered together.
We can note immediately that this equation converts the genic variance of one RF population (the focal parent) into the genic variance of another RF population (the equivalent), which presages things to come. Notice that this genic variance absorbs several genetical terms, involving not only the focal parent genic variance, but also cov(a,d), average allele substitution effect (α), gene-model homozygote effect (a), and much of the gene-model heterozygote variance (σ2d) Footnote 3. Definitely, this is not simple in terms of the gene-model interpretation; but this difficulty extends to the widely accepted classical (RF) genic variance as well.
(ii) Dominance variance

After substituting previous definitions of σ2D and σ2d this becomes:

As previously, it is convenient to leave the σ2d terms separated.
Once again, we have a conversion equation from one RF (parental) to another RF (hybrid equivalent). Notice that this dominance variance collects together all of the gene-model heterozygote variance (σ2d) not absorbed into the genic variance. It can be shown that this occurs also in the classical definition of dominance variance.
(iii) Genotypic variance
Upon accumulating eqns (5) and (7), four cancellations occur (terms 2, 3, 4 and 5 of eqn 7 with respective terms 7, 8, 9 and 10 of eqn 5) and one half-cancellation occurs (the two last terms), leading to the RF genotypic variance:

Comparison of eqn (9) with eqn (2) reveals that the genotypic variance (σ2G) of this RF population is identical with that of the allogamous F2. That is, we have now proved that these two populations are the same for yet another key property. An implication is that the allogamous F2 is an origin of RF populations. In the next section we will also make a comparison against conventionally obtained RF variances based on , which will affirm this result.
Genic and dominance variances
Having shown that the allogamous F2 and the equivalent RF are the same in several key properties, including the genotypic variance, it is obvious that the genic (σ2A) and dominance (σ2D) components of the RF population are also those of the allogamous F2. Furthermore, these two components account for all of the genotypic variance, as expected from the classical RF variances (e.g. Falconer, 1981). Even some forms of epistasis may be included as part of the dominance/overdominance specifications. Therefore, eqn (5) or eqn (3) defines the genic variance of the allogamous F2 (as well as of the equivalent RF), and eqn (7) or eqn (8) defines its dominance variance. These are visualized in Fig. 4 for our partial dominance example, where they are compared with the overall genotypic variance as well. Both the complementary opposite and the fixed p2=½ parental examples are depicted.
We noted earlier that complementary opposites genotypic variance was constant across all p1, owing to the result that =½ in this situation. We see that the same outcome applies to the genic (σ2A) and dominance (σ2D) variances as well. The fixed p2=½ case reveals that the genic and dominance variances curve-shapes are reminiscent of a segment of the curves for classical RF populations. Indeed, this is exactly the situation. If we remember that the x-axis in these graphs is p1 and not , and that only a segment of the possible spectrum can result from our example parentage, we then can observe the fixed p2=½ curves as a segment of the entire RF distribution based on its own p. This is shown in Fig. 5, where classical RF variances for our examples are graphed. The x-axis is now pRF (i.e. in our present context). The ‘window’ between the two vertical lines shows the same variances as in Fig. 4 for the fixed p2=½ case; and the vertical dashes at p=0.5 show where lie the variances for the complementary opposites case. This brings all of our present variances into line with the more familiar RF variances.
Genotypic (VG), genic (VA) and dominance (VD) variances of a classical random fertilizing (RF) population across the Universe of allele frequency p, for partial dominance (d=7.5 and a=10). Allogamous F2 form subsets of this Universe in which p is equivalent to from a cross between a focal parent (with A1 frequency p1), and an other parent (with p2 defined as an offset y from p1). The values marked at p=0.5 are those of the F2 in which the other parent is a complementary opposite to the focal parent (over all p1); while the window between the vertical lines is the sub-Universe for all p1 crossed with fixed p2=½. These are actually the same variances as shown in Fig. 4, where, however, the x-axis is p1 rather than .
Overdominance (d > a) has a marked effect on both genic and dominance variances. The dominance variance generally increases with increasing overdominance. However, the effect on the genic variance is more dynamic. For complementary opposites crosses, overdominance does not alter the genic variance, which is constant for all p1 and d. For other kinds of parental relationships, the genic variance rises with increasing overdominance for p1 < ½, and drops with increasing overdominance for p1 > ½. With extreme overdominance, the genic variance is zero for some p1.
Heritabilities
As all the genetical variances of the allogamous F2 and the RF population are identical, we can discuss heritabilities for both. The broad-sense heritability (h2B=σ2G/σ2P, where σ2P=σ2G+σ2E, and σ2E=25 in the examples) generally increases with increasing overdominance. For other parent fixed p2 crosses (as opposed to complementary opposites), there is a decline in broad-sense heritability as p1 increases.
Narrow-sense heritabilities (h2N=σ2A/σ2P) are of greater interest than broad-sense because of their prominence in selection theory, and because they exist for this F2 case. Those for the complementary opposites crosses are constant across all levels of p1, as expected from the constancy of the genic variances. Notice, however, that the level of this heritability drops with increasing dominance, because of the rising genotypic variance against the constancy of the genic variance (see earlier paragraph). For cases involving fixed other-parent p2, the levels of heritability have various patterns which reflect the underlying patterns of the genic variance (see earlier paragraph). Generally for these F2s, h2N is higher for p1 < ½, indicating that genetic advance will be greater in such parent combinations. As noted earlier, some combinations of a, d and p1 lead to zero genic variance, and hence zero h2N and zero genetic advance from selection.
Discussion
Randomly fertilized populations
Quantitative genetics is built largely around the randomly fertilized (RF) population, or its inbred derivatives. Also, it is often an added implication that the population is one RF gamodeme in which the frequencies of alleles are uniform over the entire area of the population (i.e. panmixia). Research on gene-flow (Levin and Kerster, 1974) has revealed that such a uniform one-gamodeme view is often unreasonable. For instance, of the examples tabulated in Richards (1986) (Table 5.10, p. 179), five-eighths of the examples definitely were not panmictic. However, an alternative realistic approach exists, i.e. to consider the population as a bulk of small dispersing RF gamodemes (Wright, 1943, 1946). This fits well with the natural evidence cited above. Nevertheless, we have shown here that the allogamous F2 is a RF population, and that the assumption to this effect is valid! Furthermore, we have discovered a plausible origin for RF populations in the allogamous F2 from hybrid bulks. We should realize that this RF may not necessarily be panmictic, however, and certainly not with the passage of subsequent generations when it will almost certainly become dispersed into smaller RF gamodemes.
Also, we now have the equations for converting one RF population (the focus parent) into another RF population (the derivative allogamous F2). Such re-construction of RF populations via hybridization would be expected to occur commonly in natural genetics; and it is a frequently planned activity in plant breeding.
Inbreeding
It is widely assumed that a RF population has zero inbreeding. This is largely because of its being the philosophical base of Quantitative Genetics for which it may be defined as the “natural” reference for a definition of inbreeding. The problem is that, until now, we had no clear view of a natural history of which RF populations were a part. However, this paper has demonstrated that an allogamous F2 is an origin for RF populations, thereby providing an external reference by which its inbreeding can be measured. The sexual pathway from F1 to F2 unequivocally shows that the natural reference population for the F2 is its own F1; which, in turn, has its properties set by the parents which crossed to produce it. This paper has derived the inbreeding of the allogamous F2 (and equivalent RF population) with respect to that F1. On that basis it has considerable inbreeding (see Fig. 1), the value of which depends on the relatives mixture arising from the values of p1 and p2 in the original parents. We have already noted that, if we adopt the RF population as the reference base, then inbreeding is zero for the allogamous F2; conversely, if we adopt the parental mean heterozygosity as the base, then this F2 is outbred (with negative φ).
Another issue is that it is difficult to define inbreeding in terms of full-sib, half-sib or other relationships. These terms strictly apply only to the progeny of individual parents, and not to the bulk progeny of parental populations. The proportions of full-sib, half-sib, cousin-like and backcross-like progeny outcomes within the F2 will vary considerably according to the original parent’s p1 or p2, making it difficult to resolve the inbreeding by this approach. The method used here (based on the net level of heterozygosity) is immediately exact and straightforward, and permits ready comparison with other kinds of populations, including all the classical situations mentioned above. For the complementary opposites, we have already noted that φ → ½, the level for selfing (generation 1), as p1 → 1 (or 0). And for most crossing systems, there is a p1 region for which φ → 0. The equivalent of full-sib (generation 1) (φ → 0.25) and of half-sib (generation 1) (φ → 0.125) inbreeding can also be observed at different p1 depending on the crossing system (i.e. the relativity of p1 and p2).
Selection vs. hybrid vigour
Genetic advance (ΔG) from forward selection increases with larger phenotypic variance and higher narrow-sense heritability (e.g. Falconer, 1981), as well as with stronger selection pressure. We now have the equations for these quantities in the allogamous F2, which enables us to estimate the relative effects of forward selection, hybrid vigour and inbreeding depression. In an earlier paper, similar properties were presented for the F1 (Gordon, 1999): where it was noted that maximum hybrid vigour arose from crosses with extreme p1, whereas maximum selection advance arose from central values of p1. With complementary opposites crosses, all F2, irrespective of p1, will have the same genetic advance, as discussed earlier for genic variances. With F2 from fixed p2 crosses, greater selection advance occurs asymmetrically, for noncentral p1. The mean, cumulative genetic advance, and vigour for selection pressure of P=0.1 are shown in Fig. 6, using individual selection with both sexes equally selected, and with partial dominance (a=10, d=7.5). A complementary opposites cross, with p1=0.05 is shown in Fig. 6(a), while a similar cross with p1=0.45 is shown in Fig. 6(b). Concatenating the previous F1 results with the present results, we would expect considerable hybrid vigour followed by some selection advance in the first case. In the second case, we expect minimum vigour followed by optimum selection advance.
Generation performance from focus parent (P1), through hybrid (F1) and allogamous F2 (F2) to the allogamous F3, with individual selection (P=0.1) at F1 → F2 and F2 → F3, under the partial dominance and environment variance defined previously. The mean and its components [accumulated selection advance (delta−G), and hybrid vigour from the mid-parent (vigour)] are given for two parental situations: (a) focal p1=0.05, and (b) p1=0.45. The other parent in each case is a complementary opposite.
In (a), hybrid vigour is observed, but it is followed by noticeable inbreeding depression from F1 → F2. Selection begins also at this stage, but it barely offsets the effects of the depression. Finally, in selecting F2 → F3, some real advance is made from the selection. With the case in (b), both vigour and depression are trivial, and both stages of selection yield relatively large advances. The final result at F3 is much better in case (b) than in case (a). These results are caused solely by the different properties of F1 and/or F2 for different regions of the p1 range. It appears that, in these examples, selection was more effective than hybrid vigour.
Where overdominance occurs, hybrid vigour is more conspicuous, but so is subsequent depression. Selection advance also is greater with overdominance, and always overcomes the depression to make subsequent substantial advance. In addition, if we utilize dispersion by employing selection strategies such as Combined Selection (the best individuals from the best lines), the advantage to selection becomes much greater still.
Notes
The following relations have been used to resolve these equations:
Because these SS are based on frequencies rather than counts, they equate immediately to mean-squares in fact.
Define the gene-model heterozygote variance as σ2d=2pqd2. Also define the gene-model homozygote variance as σ2a=2pqa2.
References
Allard, R. W. (1960). Principles of Plant Breeding. Wiley & Sons, New York.
Falconer, D. S. (1981). Introduction of Quantitative Genetics, 2nd edn. Longman, New York.
Gordon, I. L. (1999). Quantitative genetics of intraspecies hybrids. Heredity, 83: 757–764.
Levin, D. A. and Kerster, H. W. (1974). Gene flow in seed plants. Evol Biol, 7: 139–220.
Moore, J. N., Janik, J. (eds). (1983). Methods in Fruit Breeding. Purdue University Press, West Lafayette, IN.
Poehlman, J. M. (1979). Breeding Field Crops 2nd edn. AVI Publishing, Westport.
Richards, A. J. (1986). Plant Breeding Systems. Allen & Unwin, London.
Wright, S. (1921). Systems of mating. I. The biometrical relations between parent and offspring. Genetics, 6: 111–123.
Wright, S. (1943). Isolation by distance. Genetics, 28: 114–138.
Wright, S. (1946). Isolation by distance under diverse systems of mating. Genetics, 31: 39–59.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gordon, I. Quantitative genetics of allogamous F2: an origin of randomly fertilized populations. Heredity 85, 43–52 (2000). https://doi.org/10.1046/j.1365-2540.2000.00716.x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00716.x
Keywords
This article is cited by
-
Refinements to the partitioning of the inbred genotypic variance
Heredity (2003)