Abstract
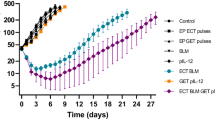
In vivo electroporation has been used to efficiently deliver drugs and ‘therapeutic’ genes to tumors, including melanoma lesions. This study reports on the effect of intratumoral delivery of an optimized DNA plasmid expressing interleukin-15 (pIL-15) on established murine melanoma tumors. IL-15 has been demonstrated to have a pivotal role in the function of memory CD8+ T cells and natural killer cells, which are critical for tumor immunosurveillance. In this study, C57BL/6 mice were injected with B16.F10 melanoma cells and randomized into different experimental groups: untreated (P−V−E−), treated with pIL-15 (P+) or backbone plasmid (V+), with or without electroporation (E+ or E−). Treatment was performed intratumorally with 50 μg of plasmid on days 0, 4 and 7 and tumor volume/size, tumor regression and long-term survival were measured. At day 100 after initiation of treatment, the percentage of mice surviving with complete tumor regression in the P−V+E+, P+V−E−, P+V−E+ and P−V−E− treatment groups were 0, 12.5, 37.5 and 0%, respectively. These results demonstrate the ability of pIL-15 to mediate B16 melanoma regression, with the effect being significantly enhanced by electroporative delivery. This is the first description of the ability of a naked DNA plasmid expressing IL-15 to alone mediate complete regression of B16 melanoma tumors and underscores the potential clinical use of these plasmids for the treatment of malignant tumors when delivered with in vivo electroporation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Andre F, Mir LM . DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Therapy 2004; 11(Suppl 1): S33–S42.
Lohr F, Lo DY, Zaharoff DA, Hu K, Zhang X, Li Y et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation. Cancer Res 2001; 61: 3281–3284.
Lucas ML, Heller L, Coppola D, Heller R . IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther 2002; 5: 668–675.
Heller L, Ugen K, Heller R . Electroporation for targeted gene transfer. Expert Opin Drug Deliv 2005; 2: 255–268.
Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA . Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J 1996; 15: 4928–4939.
Diab A, Cohen AD, Alpdogan O, Perales MA . IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy 2005; 7: 23–35.
Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8T cells that are partially independent of CD4T cell help. J Immunol 2005; 175: 112–123.
Heller LC, Coppola D . Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Therapy 2002; 9: 1321–1325.
Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP . Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA 2003; 100: 3392–3397.
Munger W, DeJoy SQ, Jeyaseelan Sr R, Torley LW, Grabstein KH, Eisenmann J et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol 1995; 165: 289–293.
Acknowledgements
We acknowledge the assistance of Chuanhai Cao in generating the necessary amounts of the control and IL-15-expressing plasmids. MAK is supported by NIH postdoctoral fellowship F32AI054152. DBW is supported by an IPCP (Integrated Preclinical/Clinical Program) grant from the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ugen, K., Kutzler, M., Marrero, B. et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther 13, 969–974 (2006). https://doi.org/10.1038/sj.cgt.7700973
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cgt.7700973
This article is cited by
-
Genome editing via non-viral delivery platforms: current progress in personalized cancer therapy
Molecular Cancer (2022)
-
Cytosolic DNA Sensor Upregulation Accompanies DNA Electrotransfer in B16.F10 Melanoma Cells
Molecular Therapy - Nucleic Acids (2016)
-
Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model
BMC Cancer (2015)
-
Intramuscular delivery of heterodimeric IL-15 DNA in macaques produces systemic levels of bioactive cytokine inducing proliferation of NK and T cells
Gene Therapy (2015)
-
Endoglin (CD105) Silencing Mediated by shRNA Under the Control of Endothelin-1 Promoter for Targeted Gene Therapy of Melanoma
Molecular Therapy - Nucleic Acids (2015)