Abstract
Aim:
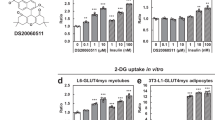
To establish the mechanism responsible for the stimulation of glucose uptake by Astragalus polysaccharide (APS), extracted from Astragalus membranaceus Bunge, in L6 myotubes in vitro.
Methods:
APS-stimulated glucose uptake in L6 myotubes was measured using the 2-deoxy-[3H]-D-glucose method. The adenine nucleotide contents in the cells were measured by HPLC. The phosphorylation of AMP-activated protein kinase (AMPK) and Akt substrate of 160 kDa (AS160) was examined using Western blot analysis. The cells transfected with 4P mutant AS160 (AS160-4P) were constructed using gene transfer approach.
Results:
Treatment of L6 myotubes with APS (100−1600 μg/mL) significantly increased glucose uptake in time- and concentration-dependent manners. The maximal glucose uptake was reached in the cells treated with APS (400 μg/mL) for 36 h. The APS-stimulated glucose uptake was significantly attenuated by pretreatment with Compound C, a selective AMPK inhibitor or in the cells overexpressing AS160-4P. Treatment of L6 myotubes with APS strongly promoted the activation of AMPK. We further demonstrated that either Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) or liver kinase B1 (LKB1) mediated APS-induced activation of AMPK in L6 myotubes, and the increased cellular AMP: ATP ratio was also involved. Treatment of L6 myotubes with APS robustly enhanced the phosphorylation of AS160, which was significantly attenuated by pretreatment with Compound C.
Conclusion:
Our results demonstrate that APS stimulates glucose uptake in L6 myotubes through the AMP-AMPK-AS160 pathway, which may contribute to its hypoglycemic effect.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP . The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000–7.
Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, et al. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest 1988; 82: 486–94.
DeFronzo RA, Bonadonna RC, Ferrannini E . Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992; 15: 318–68.
Misra P, Chakrabarti R . The role of AMP kinase in diabetes. Indian J Med Res 2007; 125: 389–98.
Hardie DG, Scott JW, Pan DA, Hudson ER . Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 2003; 546: 113–20.
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996; 271: 27879–87.
Stein SC, Woods A, Jones NA, Davison MD, Carling D . The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 2000; 345: 437–43.
Hardie DG . AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008; 32: S7–12.
Merrill GF, Kurth EJ, Hardie DG, Winder WW . AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 1997; 273: E1107–12.
Sakamoto K, Holman GD . Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 2008; 295: E29–37.
Zerial M, McBride H . Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2: 107–17.
Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 2007; 5: 293–303.
Zeigerer A, McBrayer MK, McGraw TE . Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell 2004; 15: 4406–15.
Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H . Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 2005; 54: 1692–7.
Wang N, Zhang DL, Mao XQ, Zou F, Jin H, Ou-Yang JP . Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol Cell Endocrinol 2009; 307: 89–98.
Zou F, Mao XQ, Wang N, Liu J, Ou-Yang JP . Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol Sin 2009; 30: 1607–15.
Tanishita T, Shimizu Y, Minokoshi Y, Shimazu T . The beta3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J Biochem 1997; 122: 90–5.
Hutchinson DS, Bengtsson T . alpha1A-adrenoceptors activate glucose uptake in L6 muscle cells through a phospholipase C-, phosphatidylinositol-3 kinase-, and atypical protein kinase C-dependent pathway. Endocrinology 2005; 146: 901–12.
Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, et al. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem 2003; 278: 34003–10.
Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N . Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem 2005; 280: 32081–9.
Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 2003; 278: 14599–602.
Hardie DG . Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179–83.
Bruss MD, Arias EB, Lienhard GE, Cartee GD . Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 2005; 54: 41–50.
Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006; 55: 2051–8.
Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D . Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 2006; 281: 32207–16.
Russell RR 3rd, Bergeron R, Shulman GI, Young LH . Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 1999; 277: H643–9.
Wu Y, Ou-Yang JP, Wu K, Wang Y, Zhou YF, Wen CY . Hypoglycemic effect of Astragalus polysaccharide and its effect on PTP1B. Acta Pharmacol Sin 2005; 26: 345–52.
Yeh LA, Lee KH, Kim KH . Regulation of rat liver acetyl-CoA carboxylase. Regulation of phosphorylation and inactivation of acetyl-CoA carboxylase by the adenylate energy charge. J Biol Chem 1980; 255: 2308–14.
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004; 113: 274–84.
Davies SP, Helps NR, Cohen PT, Hardie DG . 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 1995; 377: 421–5.
Wu Y, Song P, Xu J, Zhang M, Zou MH . Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 2007; 282: 9777–88.
Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006; 55: 2067–76.
Acknowledgements
We are especially grateful to Dr Laurie J GOODYEAR (Joslin Diabetes Center, Boston, MA, USA), Dr Takahiro NAGASE (Kazusa DNA Research Institute, Chiba, Japan), and Dr Jun-ichi MIYAZAKI (Osaka University Medical School, Osaka, Japan) for providing valuable materials.
This work was supported by the National Natural Science Foundation of China (No 30771023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary table is available at the Acta Pharmacologica Sinica website.
Supplementary information
Supplementary Table 1
Adenine nucleotide contents and ratio changes following various treatments. (DOC 25 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Zhang, Jf., Lu, Jz. et al. Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacol Sin 34, 137–145 (2013). https://doi.org/10.1038/aps.2012.133
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.133
Keywords
This article is cited by
-
Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance
Molecular and Cellular Biochemistry (2017)
-
Selenium-enriched exopolysaccharides improve skeletal muscle glucose uptake of diabetic KKAy mice via AMPK pathway
Journal of Physiology and Biochemistry (2014)
-
RETRACTED ARTICLE: Astragalus saponins affect proliferation, invasion and apoptosis of gastric cancer BGC-823 cells
Diagnostic Pathology (2013)
-
Danthron activates AMP-activated protein kinase and regulates lipid and glucose metabolism in vitro
Acta Pharmacologica Sinica (2013)