Abstract
Aim:
To investigate the effects of bornyl caffeate discovered in several species of plant on human breast cancer cells in vitro and the underlying mechanisms.
Methods:
Human breast cancer cell line MCF-7 and other tumor cell lines (T47D, HepG2, HeLa, and PC12) were tested. Cell viability was determined using MTT assay, and apoptosis was defined by monitoring the morphology of the nuclei and staining with Annexin V-FITC. Mitochondrial membrane potential (MMP) was measured using JC-1 under fluorescence microscopy. Intracellular reactive oxygen species (ROS) were assessed by flow cytometry. The expression of apoptosis-associated proteins was determined by Western blotting analysis.
Results:
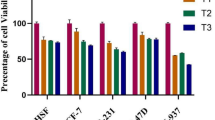
Bornyl caffeate (10, 25, and 50 μmol/L) suppressed the viability of MCF-7 cells in dose- and time-dependent manners, but neither caffeic acid nor borneol showed cytotoxicity at a concentration of 50 μmol/L. Bornyl caffeate also exerted cytotoxicity to HepG2, Hela, T47D, and PC12 cells. Bornyl caffeate dose-dependently induced apoptosis of MCF-7 cells, increased the expression of Bax and decreased the expression of Bcl-xl, resulting in the disruption of MMP and subsequent activation of caspase-3. Moreover, bornyl caffeate triggered the formation of ROS and activated p38 and c-Jun JNK. In MCF-7 cells, the cytotoxicity of bornyl caffeate was significantly attenuated by SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), z-VAD (pan-caspase inhibitor) or the thiol antioxidant L-NAC.
Conclusion:
Bornyl caffeate exerts non-selective cytotoxicity against cancer cells of different origin in vitro. The compound induces apoptosis in human breast cancer MCF-7 cells via the ROS- and JNK-mediated pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hetts SW . To die or not to die. JAMA 1998; 279: 300–7.
Zhou Y, ZHANG WP . Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats1. Acta pharmacol Sin 2006; 27: 1103–10.
Bamford M, Walkinshaw G, Brown R . Therapeutic applications of apoptosis research. Exp Cell Res 2000; 256: 1–11.
Kaufmann SH, Earnshaw WC . Induction of apoptosis by cancer chemotherapy. Exp Cell Res 2000; 256: 42–9.
Johnson GL, Lapadat R . Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–2.
Wada T, Penninger JM . Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004; 23: 2838–49.
Lewis TS, Shapiro PS, Ahn NG . Signal transduction through MAP kinase cascades. Adv Cancer Res 1998; 74: 49–139.
Band CJ, Posner BI . Phosphatidylinositol 3′-kinase and p70s6k are required for insulin but not bisperoxovanadium 1,10-phenanthroline [bpV (phen)] inhibition of insulin-like growth factor binding protein gene expression. J Biol Chem 1997; 272: 138–45.
Grammer TC, Blenis J . Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene 1997; 14: 1635–42.
Wang X, Martindale JL, Liu Y, Holbrook NJ . The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J 1998; 333: 291–300.
Traverse S, Gomez N, Paterson H, Marshall C, Cohen P . Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J 1992; 288: 351–5.
Ishikawa Y, Kitamura M . Dual potential of extracellular signal-regulated kinase for the control of cell survival. Biochem Biophys Res Commun 1999; 264: 696–701.
Obata T, Brown GE, Yaffe MB . MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med 2000; 28: N67–77.
Kang KW, Ryu JH, Kim SG . The essential role of phosphatidylinositol 3-kinase and of p38 mitogen-activated protein kinase activation in the antioxidant response element-mediated rGSTA2 induction by decreased glutathione in H4IIE hepatoma cells. Mol Pharmacol 2000; 58: 1017–25.
Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 1996; 15: 2760–70.
Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem 1997; 272: 30122–8.
Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N . MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci U S A 1999; 96: 15127–32.
Harada J, Sugimoto M . Activation of caspase-3 in β-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res 1999; 842: 311–23.
Davis RJ . Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52.
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X . Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15: 269–90.
Borner C, Monney L . Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ 1999; 6: 497–507.
Leist M, Jaattela M . Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001; 2: 589–98.
Maldonado E, Ramírez Apan M, Perez-Castorena AL . Anti-inflammatory activity of phenyl propanoids from Coreopsis mutica var. mutica. Planta Med 1998; 64: 660–1.
Setzer WN, Setzer MC, Bates RB, Nakkiew P, Jackes BR, Chen L, et al. Antibacterial hydroxycinnamic esters from Piper caninum from Paluma, north Queensland, Australia. The crystal and molecular structure of (+)-bornyl coumarate. Planta Med 1999; 65: 747–9.
Chen YC, Liao CH, Chen IS . Lignans, an amide and anti-platelet activities from Piper philippinum. Phytochemistry 2007; 68: 2101–11.
Ogungbe IV, Crouch RA, Haber WA, Setzer WN . Phytochemical investigation of verbesina turbacensis kunth: trypanosome cysteine protease inhibition by (−)-bornyl esters. Nat Prod Commun 2010; 5: 1161–6.
Steinbrecher T, David A, Labahn A . A multistep approach to structure-based drug design: Studying ligand binding at the human neutrophil elastase. J Med Chem 2006; 49: 1837–44.
Xia C, Li H, Hu W . Synthesis of trans-caffeate analogues and their bioactivities against HIV-1 integrase and cancer cell lines. Bioorg Med Chem Lett 2008; 18: 6553–7.
Cha JD, Jeong MR, Jeong SI, Lee KY . Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens. J Microbiol Biotechnol 2007; 17: 858–64.
Mulyaningsih S, Youns M, El-Readi MZ, Ashour ML, Nibret E, Sporer F, et al. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J Pharm Pharmacol 2010; 62: 1037–44.
Slamenova D, Horvathova E, Wsolova L, Sramkova M, Navarova J . Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat Res 2009; 677: 46–52.
Kim Y, Cerbo R, Hah C, Bahn K, Kim J, Ha Y . Growth inhibition of osteosarcoma cell MG-63 by a mixture of trans, trans conjugated linoleic acid isomers: possible mechanistic actions. J Food Sci 2008; 73: T7–15.
Jasinski P, Zwolak P, Isaksson Vogel R, Bodempudi V, Terai K, Galvez J, et al. MT103 inhibits tumor growth with minimal toxicity in murine model of lung carcinoma via induction of apoptosis. Invest New Drugs 2011; 29: 846–52.
Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW . Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol 1994; 48: 595–608.
Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, et al. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem 2003; 51: 3302–8.
Orban Z, Mitsiades N, Burke Jr TR, Tsokos M, Chrousos GP . Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation 2000; 7: 99–105.
Kwon KH, Barve A, Yu S, HUANG MT, KONG ANT . Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models1. Acta Pharmacol Sin 2007; 28: 1409–21.
Zhang L, Ji Z . Synthesis, antiinflammatory and anticancer activity of cinnamic acids, their derivatives and analogues. Yao Xue Xue Bao 1992; 27: 817–23.
Su Z, Lin J, Grunberger D, Fisher PB . Growth suppression and toxicity induced by caffeic acid phenethyl ester (CAPE) in type 5 adenovirus-transformed rat embryo cells correlate directly with transformation progression. Cancer Res 1994; 54: 1865–70.
Chen JH, Shao Y, Huang MT, Chin CK, Ho CT . Inhibitory effect of caffeic acid phenethyl ester on human leukemia HL-60 cells. Cancer Lett 1996; 108: 211–4.
Fiuza S, Gomes C, Teixeira L, Girão da Cruz M, Cordeiro M, Milhazes N, et al. Phenolic acid derivatives with potential anticancer properties — a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem 2004; 12: 3581–9.
da Cunha FM, Duma D, Assreuy J, Buzzi FC, Niero R, Campos MM, et al. Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic Res 2004; 38: 1241–53.
Ujibe M, Kanno S, Osanai Y, Koiwai K, Ohtake T, Kimura K, et al. Octylcaffeate induced apoptosis in human leukemia U937 cells. Biol Pharm Bull 2005; 28: 2338–41.
Nagaoka T, Banskota AH, Tezuka Y, Harimaya Y, Koizumi K, Saiki I, et al. Inhibitory effects of caffeic acid phenethyl ester analogues on experimental lung metastasis of murine colon 26-L5 carcinoma cells. Biol Pharm Bull 2003; 26: 638–41.
Etzenhouser B, Hansch C, Kapur S, Selassie CD . Mechanism of toxicity of esters of caffeic and dihydrocaffeic acids. Bioorg Med Chem 2001; 9: 199–209.
Rong J, Tilton R, Shen J, Ng KM, Liu C, Tam PKH, et al. Genome-wide biological response fingerprinting (BioReF) of the Chinese botanical formulation ISF-1 enables the selection of multiple marker genes as a potential metric for quality control. J Ethnopharmacol 2007; 113: 35–44.
Qi H, Siu SO, Chen Y, Han Y, Chu IK, Tong Y, et al. Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1. Chem Biol Interact 2010; 183: 380–9.
Qi H, Chen B, Le XC, Rong J . Concomitant induction of heme Oxygenase-1 attenuates the cytotoxicity of arsenic species from lumbricus extract in human liver HepG2 cells. Chem Biodivers 2012; 9: 739–54.
Choi IY, Lee SJ, Ju C, Nam W, Kim HC, Ko KH, et al. Protection by a manganese porphyrin of endogenous peroxynitrite-induced death of glial cells via inhibition of mitochondrial transmembrane potential decrease. Glia 2000; 31: 155–64.
Rong J, Cheung C, Lau A, Shen J, Tam P, Cheng YC . Induction of heme oxygenase-1 by traditional Chinese medicine formulation ISF-1 and its ingredients as a cytoprotective mechanism against oxidative stress. Int J Mol Med 2008; 21: 405–11.
Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY . Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem 2001; 49: 5615–9.
Sanmartín C, Plano D, Sharma AK, Palop JA . Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci 2012; 13: 9649–72.
Thornberry NA, Lazebnik Y . Caspases: enemies within. Science 1998; 281: 1312–6.
Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells — A mechanism of immune evasion? Nature Med 1996; 2: 1361–6.
Lee JC, Son YO, Pratheeshkumar P, Shi X . Oxidative stress and metal carcinogenesis. Free Radic Biol Med 2012; 53:742–57.
Klaunig JE, Kamendulis LM, Hocevar BA . Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 2010; 38: 96–109.
Fruehauf JP, Meyskens FL . Reactive oxygen species: a breath of life or death? Clin Cancer Res 2007; 13: 789–94.
Trachootham D, Alexandre J, Huang P . Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–91.
Benhar M, Engelberg D, Levitzki A . ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 2002; 3: 420–5.
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B . Role of BAX in the apoptotic response to anticancer agents. Science 2000; 290: 989–92.
Raza H, John A, Benedict S . Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol 2011; 668: 15–24.
Eruslanov E, Kusmartsev S . Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 2010; 594: 57–72.
Mason W . Fluorescent and luminescent probes for biological activity: a practical guide to technology for quantitative real-time analysis. Second Edition ed. London, UK Academic Press; 1999.
Rohlena J, Dong LF, Ralph SJ, Neuzil J . Anticancer drugs targeting the mitochondrial electron transport chain. Antioxid Redox Signal 2011; 15: 2951–74.
Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R . A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res 2005; 65: 1035–44.
Liu H, Liu Y, Liu Y, Xu A, Young CYF, Yuan H, et al. A novel anticancer agent, retigeric acid B, displays proliferation inhibition, S phase arrest and apoptosis activation in human prostate cancer cells. Chem Biol Interact 2010; 188: 598–606.
Chang L, Karin M . Mammalian MAP kinase signalling cascades. Nature 2001; 410: 37–40.
Kyriakis JM, Avruch J . Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81: 807–69.
Seki E, Brenner DA, Karin M . A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 2012; 143: 307–20.
Kelkel M, Cerella C, Mack F, Schneider T, Jacob C, Schumacher M, et al. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 2012; 33: 2162–71.
Kim DE, Min K, Kim JS, Kwon TK . High-mobility group box-1 protein induces mucin 8 expression through the activation of the JNK and PI3K/Akt signal pathways in human airway epithelial cells. Biochem Biophys Res Commun 2012; 421: 436–41.
Acknowledgements
This work was supported by the Seed Funding Programme for Basic Research, University of Hong Kong (Project 201111159212, to JR) and the National Natural Science Foundation of China, Specialized Research Fund for the Doctoral Program of Higher Education on 2011 (project 20106101110001, to XZ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary figure is available at the Acta Pharmacologica Sinica website.
Supplementary information
Figure S1
Cytotoxicity of bornylcaffeate indifferentcell line. (DOCX 159 kb)
Rights and permissions
About this article
Cite this article
Yang, Cb., Pei, Wj., Zhao, J. et al. Bornyl caffeate induces apoptosis in human breast cancer MCF-7 cells via the ROS- and JNK-mediated pathways. Acta Pharmacol Sin 35, 113–123 (2014). https://doi.org/10.1038/aps.2013.162
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.162
Keywords
This article is cited by
-
In vitro evaluation of p-coumaric acid and naringin combination in human epidermoid carcinoma cell line (A431)
Medical Oncology (2023)
-
TFEB, a master regulator of autophagy and biogenesis, unexpectedly promotes apoptosis in response to the cyclopentenone prostaglandin 15d-PGJ2
Acta Pharmacologica Sinica (2022)
-
PHD-2 activation: a novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
New benzimidazole acridine derivative induces human colon cancer cell apoptosis in vitro via the ROS-JNK signaling pathway
Acta Pharmacologica Sinica (2015)
-
Amygdalin isolated from Semen Persicae (Tao Ren) extracts induces the expression of follistatin in HepG2 and C2C12 cell lines
Chinese Medicine (2014)