Abstract
Aim:
To examine the therapeutic effects and underlying mechanisms of DZ2002, a reversible S-adenosyl-L-homocysteine hydrolase (SAHH) inhibitor, on lupus-prone female NZB×NZW F1 (NZB/W F1) mice.
Methods:
Female NZB/W F1 mice were treated orally with DZ2002 (0.5 mg·kg−1·d−1) for 11 weeks, and the proteinuria level and body weight were monitored. After the mice ware euthanized, serum biochemical parameters and renal damage were determined. Splenocytes of NZB/W F1 mice were isolated for ex vivo study. Toll-like receptor (TLR)-stimulated human peripheral blood mononuclear cells (PBMCs) or murine bone marrow-derived dendritic cells (BMDCs) were used for in vitro study.
Results:
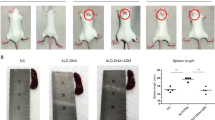
Treatment of the mice with DZ2002 significantly attenuated the progression of glomerulonephritis and improved the overall health. The improvement was accompanied by decreased levels of nephritogenic anti-dsDNA IgG2a and IgG3 antibodies, serum IL-17, IL-23p19 and TGF-β. In ex vivo studies, treatment of the mice with DZ2002 suppressed the development of pathogenic Th17 cells, significantly decreased IL-17, TGF-β, IL-6, and IL-23p19 production and impeded activation of the STAT3 protein and JNK/NF-κB signaling in splenocytes. DZ2002 (500 μmol/L) significantly suppressed TLR agonists-stimulated up-regulation in IL-6, IL-12p40, TNF-α, and IgG and IgM secretion as well as in HLA-DR and CD40 expression of dendritic cells among human PBMCs in vitro. DZ2002 (100 μmol/L) also significantly suppressed TLR agonists-stimulated up-regulation in IL-6 and IL-23p19 production in murine BMDCs, and prevented Th17 differentiation and suppressed IL-17 secretion by the T cells in a BMDC-T cell co-culture system.
Conclusion:
DZ2002 effectively ameliorates lupus syndrome in NZB/W F1 mice by regulating TLR signaling-mediated antigen presenting cell (APC) responses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tsokos GC . Systemic lupus erythematosus. N Engl J Med 2011; 365: 2110–21.
Wentworth J, Davies C . Systemic lupus erythematosus. Nat Rev Drug Discov 2009; 8: 103–4.
Theofilopoulos AN, Dixon FJ . Murine models of systemic lupus erythematosus. Adv Immunol 1985; 37: 269–390.
Perry D, Sang A, Yin Y, Zheng YY, Morel L . Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011; 2011: 271694.
Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ . Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006; 25: 417–28.
Hackl D, Loschko J, Sparwasser T, Reindl W, Krug AB . Activation of dendritic cells via TLR7 reduces Foxp3 expression and suppressive function in induced Tregs. Eur J Immunol 2011; 41: 1334–43.
Richez C, Blanco P, Rifkin I, Moreau JF, Schaeverbeke T . Role for toll-like receptors in autoimmune disease: the example of systemic lupus erythematosus. Joint Bone Spine 2011; 78: 124–30.
Hang L, Slack JH, Amundson C, Izui S, Theofilopoulos AN, Dixon FJ . Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med 1983; 157: 874–83.
Garrigan K, Moroni-Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, et al. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood 1996; 88: 3508–12.
Moser M, Murphy KM . Dendritic cell regulation of TH1-TH2 development. Nat Immunol 2000; 1: 199–205.
Re F, Strominger JL . Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology 2004; 209: 191–8.
Miossec P . Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum 2003; 48: 594–601.
Shih DQ, Targan SR, McGovern D . Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep 2008; 10: 568–75.
Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 2000; 43: 2455–63.
Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW . Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol 2008; 127: 385–93.
Nalbandian A, Crispin JC, Tsokos GC . Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunology 2009; 157: 209–15.
Lombardi V, Van Overtvelt L, Horiot S, Moingeon P . Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol 2009; 182: 3372–9.
Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, et al. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol 2010; 184: 1159–67.
Wolos JA, Frondorf KA, Esser RE . Immunosuppression mediated by an inhibitor of S-adenosyl-L-homocysteine hydrolase. Prevention and treatment of collagen-induced arthritis. J Immunol 1993; 151: 526–34.
Saso Y, Conner EM, Teegarden BR, Yuan CS . S-Adenosyl-L-homocysteine hydrolase inhibitor mediates immunosuppressive effects in vivo: suppression of delayed type hypersensitivity ear swelling and peptidoglycan polysaccharide-induced arthritis. J Pharmacol Exp Ther 2001; 296: 106–12.
Wolos JA, Frondorf KA, Babcock GF, Stripp SA, Bowlin TL . Immunomodulation by an inhibitor of S-adenosyl-L-homocysteine hydrolase: inhibition of in vitro and in vivo allogeneic responses. Cell Immunol 1993; 149: 402–8.
Wu QL, Fu YF, Zhou WL, Wang JX, Feng YH, Liu J, et al. Inhibition of S-adenosyl-L-homocysteine hydrolase induces immunosuppression. J Pharmacol Exp Ther 2005; 313: 705–11.
Fu YF, Wang JX, Zhao Y, Yang Y, Tang W, Ni J, et al. S-adenosyl-L-homocysteine hydrolase inactivation curtails ovalbumin-induced immune responses [Research Support, Non-US Gov't]. The Journal of pharmacology and experimental therapeutics 2006; 316: 1229–37.
Lawson BR, Manenkova Y, Ahamed J, Chen X, Zou JP, Baccala R, et al. Inhibition of transmethylation down-regulates CD4 T cell activation and curtails development of autoimmunity in a model system. J Immunol 2007; 178: 5366–74.
Fu YF, Zhu YN, Ni J, Zhong XG, Tang W, Re YD, et al. A reversible S-adenosyl-L-homocysteine hydrolase inhibitor ameliorates experimental autoimmune encephalomyelitis by inhibiting T cell activation. The Journal of pharmacology and experimental therapeutics 2006; 319: 799–808.
Tardif V, Manenkova Y, Berger M, Hoebe K, Zuo JP, Yuan C, et al. Critical role of transmethylation in TLR signaling and systemic lupus erythematosus. Clin Immunology 2013; 147: 133–43.
Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 2008; 58: 1433–44.
Tao X, Fan F, Hoffmann V, Longo NS, Lipsky PE . Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res Ther 2006; 8: R24.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–55.
Akahoshi M, Nakashima H, Tanaka Y, Kohsaka T, Nagano S, Ohgami E, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum 1999; 42: 1644–8.
Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV . Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med 2006; 203: 789–97.
Border WA, Noble NA . Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331: 1286–92. eng.
Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, et al. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 2012; 81: 865–79.
Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282: 9358–63.
Zhai YP, Lu Q, Liu YW, Cheng Q, Wei YQ, Zhang F, et al. Over-production of nitric oxide by oxidative stress-induced activation of the TGF-beta1/PI3K/Akt pathway in mesangial cells cultured in high glucose. Acta pharmacol Sin 2013; 34: 507–14.
Jung Y, Byeon SE, Yoo DS, Lee YG, Yu T, Yang Y, et al. 8-(Tosylamino)quinoline inhibits macrophage-mediated inflammation by suppressing NF-kappaB signaling. Acta pharmacol Sin 2012; 33: 1037–46.
Bekeredjian-Ding I, Jego G . Toll-like receptors — sentries in the B-cell response. Immunology 2009; 128: 311–23.
Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ . Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med 2005; 202: 321–31.
Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol 2010; 184: 1840–8.
Afzali B, Lombardi G, Lechler RI, Lord GM . The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 2007; 148: 32–46.
Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ . Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum 2007; 56: 2936–46.
Wang QQ, Yang HZ, Liu HZ, Mi S, Zhang XW, Yan HM, et al. Interleukin-17A is involved in development of spontaneous pulmonary emphysema caused by Toll-like receptor 4 mutation. Acta Pharmacol Sin 2011; 32: 1045–54.
Yang ML, Gee AJ, Gee RJ, Zurita-Lopez CI, Khare S, Clarke SG, et al. Lupus autoimmunity altered by cellular methylation metabolism. Autoimmunity 2013; 46: 21–31.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (No 81072652, 81273524, 81273525); the National Science & Technology Major Project “New Drug Creation and Manufacturing Program”, China (2012ZX09102-101-006); and the Science & Technology Commission of Shanghai Municipality, China (11431921102).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary figures were available on the APS's website.
Supplementary information
Rights and permissions
About this article
Cite this article
He, Sj., Lin, Zm., Wu, Yw. et al. Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZB×NZW F1 mice via interference with TLR-mediated APC response. Acta Pharmacol Sin 35, 219–229 (2014). https://doi.org/10.1038/aps.2013.167
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.167
Keywords
This article is cited by
-
DZ2002 alleviates corneal angiogenesis and inflammation in rodent models of dry eye disease via regulating STAT3-PI3K-Akt-NF-κB pathway
Acta Pharmacologica Sinica (2024)
-
A novel tricyclic BTK inhibitor suppresses B cell responses and osteoclastic bone erosion in rheumatoid arthritis
Acta Pharmacologica Sinica (2021)
-
Reversible SAHH inhibitor protects against glomerulonephritis in lupus-prone mice by downregulating renal α-actinin-4 expression and stabilizing integrin-cytoskeleton linkage
Arthritis Research & Therapy (2019)
-
DZ2002 ameliorates fibrosis, inflammation, and vasculopathy in experimental systemic sclerosis models
Arthritis Research & Therapy (2019)
-
Carbon sequestration in an expanded lake system during the Toarcian oceanic anoxic event
Nature Geoscience (2017)