Abstract
Aim:
Androgen receptor (AR) antagonists have proven to be useful in the early control of prostate cancer. The aim of this study was to identify and characterize a novel β-amino-carbonyl-based androgen receptor antagonist.
Methods:
Different isomers of the β-amino-carbonyl compounds were obtained by chiral separation. The bioactivities of the isomers were evaluated by AR nuclear translocation, mammalian two-hybrid, competitive receptor binding and cell proliferation assays. The expression of genes downstream of AR was analyzed with real-time PCR. The therapeutic effects on tumor growth in vivo were observed in male SCID mice bearing LNCaP xenografts.
Results:
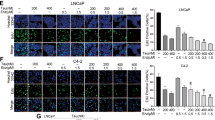
Compound 21 was previously identified as an AR modulator by the high-throughput screening of a diverse compound library. In the present study, the two isomers of compound 21, termed compounds 21-1 and 21-2, were characterized as partial AR agonists in terms of androgen-induced AR nuclear translocation, prostate-specific antigen expression and cell proliferation. Further structural modifications led to the discovery of a androgen receptor antagonist (compound 6012), which blocked androgen receptor nuclear translocation, androgen-responsive gene expression and androgen-dependent LNCaP cell proliferation. Four stereoisomers of compound 6012 were isolated, and their bioactivities were assessed. The pharmacological effects of 6012, including AR binding, androgen-induced AR translocation, NH2- and COOH-terminal interaction, growth inhibition of LNCaP cells in vitro and LNCaP xenograft growth in nude mice, were mainly restricted to isomer 6012-4 (1R, 3S).
Conclusion:
Compound 6012-4 was determined to be a novel androgen receptor antagonist with prostate cancer inhibitory activities comparable to bicalutamide both in vitro and in vivo.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29.
Blackledge GR, Cockshott ID, Furr BJ . Casodex (bicalutamide): overview of a new antiandrogen developed for the treatment of prostate cancer. Eur Urol 1997; 31: 30–9.
Kolvenbag GJ, Blackledge GR, Gotting-Smith K . Bicalutamide (Casodex) in the treatment of prostate cancer: history of clinical development. Prostate 1998; 34: 61–72.
Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol 1998; 33: 447–56.
Müderris II, Bayram F, Ozçelik B, Güven M . New alternative treatment in hirsutism: bicalutamide 25 mg/day. Gynecol Endocrinol 2002; 16: 63–6.
Colabufo NA, Pagliarulo V, Berardi F, Contino M, Inglese C, Niso M, et al. Bicalutamide failure in prostate cancer treatment: involvement of Multi Drug Resistance proteins. Eur J Pharmacol 2008; 601: 38–42.
Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009; 138: 245–56.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324: 787–90.
Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, et al. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci U S A 2005; 102: 9802–7.
Zhou C, Wu G, Feng Y, Li Q, Su H, Mais DE, et al. Discovery and biological characterization of a novel series of androgen receptor modulators. Br J Pharmacol 2008; 154: 440–50.
Ning M, Zhou C, Weng J, Zhang S, Chen D, Yang C, et al. Biological activities of a novel selective oestrogen receptor modulator derived from raloxifene (Y134). Br J Pharmacol 2007; 150: 19–28.
Bohl CE, Miller DD, Chen J, Bell CE, Dalton JT . Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem 2005; 280: 37747–54.
Sun C, Shi Y, Xu LL, Nageswararao C, Davis LD, Segawa T, et al. Androgen receptor mutation (T877A) promotes prostate cancer cell growth and cell survival. Oncogene 2006; 25: 3905–13.
Ishiguro H, Ishiguro Y, Kubota Y, Uemura H . Regulation of prostate cancer cell growth and PSA expression by angiotensin II receptor blocker with peroxisome proliferator-activated receptor gamma ligand like action. Prostate 2007; 67: 924–32.
Kang Z, Pirskanen A, Jänne OA, Palvimo JJ . Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 2002; 277: 48366–71.
Bai VU, Cifuentes E, Menon M, Barrack ER, Reddy GP . Androgen receptor regulates Cdc6 in synchronized LNCaP cells progressing from G1 to S phase. J Cell Physiol 2005; 204: 381–7.
Zhang J, Hsu B A JC, Kinseth B A MA, Bjeldanes LF, Firestone GL . Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer 2003; 98: 2511–20.
Acknowledgements
We are indebted to Ms Xiao-yan WU for technical assistance. This work was partially supported by grants from the Ministry of Health (2012ZX09304-011, 2013ZX09401003-005, 2013ZX09507001, and 2013ZX09507002), Shanghai Science and Technology Development Fund (13DZ2290300) and Thousand Talents Program in China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information is available at Acta Pharmacologica Sinica's website.
Supplementary information
Figure 1
Compound-induced androgen receptor nuclear translocation. (DOC 267 kb)
Figure 2
Cytotoxicity assay. (DOC 994 kb)
Figure 3
In vivo activity of isomer 4 of compound 6012 against LNCaP xenograft tumor in SCID mice. (DOC 833 kb)
Figure 4
Structures of 6012 and its four isomers. (DOC 65 kb)
Figure 5
ORTEP drawing of the X-ray crystal structures of 6012-2, 6012-3 and 6012-4. (DOC 79 kb)
Table 1
Binding of 72 β-amino-carbonyl analogues to androgen receptors (NA: none activity). (DOC 1161 kb)
Rights and permissions
About this article
Cite this article
Zhang, Zy., Zhu, Yh., Zhou, Ch. et al. Development of β-amino-carbonyl compounds as androgen receptor antagonists. Acta Pharmacol Sin 35, 664–673 (2014). https://doi.org/10.1038/aps.2013.201
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.201