Abstract
Aim:
Fatty acid-binding protein 4 (FABP4) plays an important role in maintaining glucose and lipid homeostasis. The aim of this study was to find new inhibitors of FABP4 for the treatment of type 2 diabetes.
Methods:
Human FABP4 protein was expressed, and its inhibitors were detected in 1,8-ANS displacement assay. The effect of the inhibitor on lipolysis activity was examined in mouse 3T3-L1 preadipocytes. The db/db mice were used to evaluate the anti-diabetic activity of the inhibitor. Molecular docking and site-directed mutagenesis studies were carried out to explore the binding mode between the inhibitor and FABP4.
Results:
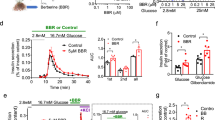
From 232 compounds tested, benzbromarone (BBR), an old uricosuric drug, was discovered to be the best inhibitor of FABP4 with an IC50 value of 14.8 μmol/L. Furthermore, BBR (25 μmol/L) significantly inhibited forskolin-stimulated lipolysis in 3T3-L1 cells. Oral administration of BBR (25 or 50 mg/kg, for 4 weeks) dose-dependently reduced the blood glucose level and improved glucose tolerance and insulin resistance in db/db mice. Molecular docking revealed that the residues Ser55, Asp76, and Arg126 of FABP4 formed important interactions with BBR, which was confirmed by site-directed mutagenesis studies.
Conclusion:
BBR is an inhibitor of FABP4 and a potential drug candidate for the treatment of type 2 diabetes and atherosclerosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Spiegelman BM, Frank M, Green H . Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem 1983; 258: 10083–9.
Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM . Adipocyte P2 gene: developmental expression and homology of 5′-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A 1986; 83: 3786–90.
Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM . Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996; 274: 1377–9.
Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS . Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 2000; 141: 3388–96.
Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci U S A 2006; 103: 6970–5.
Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 2001; 7: 699–705.
Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007; 447: 959–65.
Heel RC, Brogden RN, Speight TM, Avery GS . Benzbromarone: a review of its pharmacological properties and therapeutic use in gout and hyperuricaemia. Drugs 1977; 14: 349–66.
Cai HY, Yan GR, Zhang XD, Gorbenko O, Wang HY, Zhu WL . Discovery of highly selective inhibitors of human fatty acid binding protein 4 (FABP4) by virtual screening. Bioorg Med Chem Lett 2010; 20: 3675–9.
Zhang XD, Yan JW, Yan GR, Sun XY, Ji J, Li YM, et al. Pharmacological inhibition of diacylglycerol acyltransferase 1 reduces body weight gain, hyperlipidemia, and hepatic steatosis in db/db mice. Acta Pharmacol Sin 2010; 31: 1470–7.
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998; 19: 1639–62.
Sulsky R, Magnin DR, Huang Y, Simpkins L, Taunk P, Patel M, et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP). Bioorg Med Chem Lett 2007; 17: 3511–5.
Coe NR, Simpson MA, Bernlohr DA . Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res 1999; 40: 967–72.
Hertzel AV, Hellberg K, Reynolds JM, Kruse AC, Juhlmann BE, Smith AJ, et al. Identification and characterization of a small molecule inhibitor of Fatty Acid binding proteins. J Med Chem 2009; 52: 6024–31.
Lehmann F, Haile S, Axen E, Medina C, Uppenberg J, Svensson S, et al. Discovery of inhibitors of human adipocyte fatty acid-binding protein, a potential type 2 diabetes target. Bioorg Med Chem Lett 2004; 14: 4445–8.
Ringom R, Axen E, Uppenberg J, Lundback T, Rondahl L, Barf T . Substituted benzylamino-6-(trifluoromethyl)pyrimidin-4(1H)-ones: a novel class of selective human A-FABP inhibitors. Bioorg Med Chem Lett 2004; 14: 4449–52.
Barf T, Lehmann F, Hammer K, Haile S, Axen E, Medina C, et al. N-Benzyl-indolo carboxylic acids: Design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors. Bioorg Med Chem Lett 2009; 19: 1745–8.
LaLonde JM, Levenson MA, Roe JJ, Bernlohr DA, Banaszak LJ . Adipocyte lipid-binding protein complexed with arachidonic acid. Titration calorimetry and X-ray crystallographic studies. J Biol Chem 1994; 269: 25339–47.
Marr E, Tardie M, Carty M, Brown Phillips T, Wang IK, Soeller W, et al. Expression, purification, crystallization and structure of human adipocyte lipid-binding protein (aP2). Acta Crystallogr Sect F Struct Biol Cryst Commun 2006; 62: 1058–60.
Butler EG, Ichida T, Maruyama H, Schulte-Hermann R, Williams GM . Toxicological studies on a benzofurane derivative. II. Demonstration of peroxisome proliferation in rat liver. Toxicol Appl Pharmacol 1990; 106: 500–8.
Kunishima C, Inoue I, Oikawa T, Nakajima H, Komoda T, Katayama S . Activating effect of benzbromarone, a uricosuric drug, on peroxisome proliferator-activated receptors. PPAR Res 2007; 2007: 36092–8.
Montanari R, Saccoccia F, Scotti E, Crestani M, Godio C, Gilardi F, et al. Crystal structure of the peroxisome proliferator-activated receptor gamma (PPARgamma) ligand binding domain complexed with a novel partial agonist: a new region of the hydrophobic pocket could be exploited for drug design. J Med Chem 2008; 51: 7768–76.
Festuccia WT, Laplante M, Berthiaume M, Gelinas Y, Deshaies Y . PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 2006; 49: 2427–36.
Carmona MC, Louche K, Nibbelink M, Prunet B, Bross A, Desbazeille M, et al. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int J Obes (Lond) 2005; 29: 864–71.
WHO Drug Information: Benzbromarone and hepatitis. WHO Drug Information 2000; 14.
Lee MH, Graham GG, Williams KM, Day RO . A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients? Drug Saf 2008; 31; 643–65.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (20721003 and 81072681), the International S&T Cooperation (2010DFB73280) and the National 863 High Performance Computing Project (2012AA01A305), China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplemental Table S1 and Figure S1 are available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary Table S1
Comparison of the predicted binding free energy of BBR and FABP4 (wild type/mutants) (DOC 326 kb)
Rights and permissions
About this article
Cite this article
Cai, Hy., Wang, T., Zhao, Jc. et al. Benzbromarone, an old uricosuric drug, inhibits human fatty acid binding protein 4 in vitro and lowers the blood glucose level in db/db mice. Acta Pharmacol Sin 34, 1397–1402 (2013). https://doi.org/10.1038/aps.2013.97
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.97
Keywords
This article is cited by
-
The Effects of FABP4 on Cardiovascular Disease in the Aging Population
Current Atherosclerosis Reports (2024)
-
Uric acid‐induced pancreatic β-cell dysfunction
BMC Endocrine Disorders (2021)
-
Exogenous FABP4 interferes with differentiation, promotes lipolysis and inflammation in adipocytes
Endocrine (2020)
-
Application of target repositioning and in silico screening to exploit fatty acid binding proteins (FABPs) from Echinococcus multilocularis as possible drug targets
Journal of Computer-Aided Molecular Design (2020)
-
Metabolic functions of FABPs—mechanisms and therapeutic implications
Nature Reviews Endocrinology (2015)