Abstract
Aim:
To construct a quantitative pharmacophore model of tubulin inhibitors and to discovery new leads with potent antitumor activities.
Methods:
Ligand-based pharmacophore modeling was used to identify the chemical features responsible for inhibiting tubulin polymerization. A set of 26 training compounds was used to generate hypothetical pharmacophores using the HypoGen algorithm. The structures were further validated using the test set, Fischer randomization method, leave-one-out method and a decoy set, and the best model was chosen to screen the Specs database. Hit compounds were subjected to molecular docking study using a Molecular Operating Environment (MOE) software and to biological evaluation in vitro.
Results:
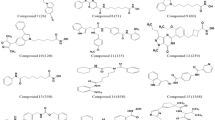
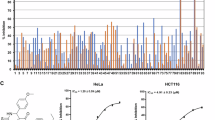
Hypo1 was demonstrated to be the best pharmacophore model that exhibited the highest correlation coefficient (0.9582), largest cost difference (70.905) and lowest RMSD value (0.6977). Hypo1 consisted of one hydrogen-bond acceptor, a hydrogen-bond donor, a hydrophobic feature, a ring aromatic feature and three excluded volumes. Hypo1 was validated with four different methods and had a goodness-of-hit score of 0.81. When Hypo1 was used in virtual screening of the Specs database, 952 drug-like compounds were revealed. After docking into the colchicine-binding site of tubulin, 5 drug-like compounds with the required interaction with the critical amino acid residues and the binding free energies <-4 kcal/mol were selected as representative leads. Compounds 1 and 3 exhibited inhibitory activity against MCF-7 human breast cancer cells in vitro.
Conclusion:
Hypo1 is a quantitative pharmacophore model for tubulin inhibitors, which not only provides a better understanding of their interaction with tubulin, but also assists in discovering new potential leads with antitumor activities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Tolomeo M, et al. Design, synthesis and structure-activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of inhibitors of tubulin polymerization. Bioorg Med Chem 2009; 17: 6862–71.
Romagnoli R, Baraldi PG, Carrion MD, Cruz-Lopez O, Tolomeo M, Grimaudo S, et al. Substituted 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]thiophene derivatives as potent tubulin polymerization inhibitors. Bioorg Med Chem 2010; 18: 5114–22.
Jiang JD, Wang Y, Roboz J, Strauchen J, Holland JF, Bekesi JG . Inhibition of microtubule assembly in tumor cells by 3-bromoacetylamino benzoylurea, a new cancericidal compound. Cancer Res 1998; 58: 2126–33.
Jordan MA, Wilson L . Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253–65.
Hu L, Li ZR, Li Y, Qu J, Ling YH, Jiang JD, et al. Synthesis and structure-activity relationships of carbazole sulfonamides as a novel class of antimitotic agents against solid tumors. J Med Chem 2006; 49: 6273–82.
Dumontet C, Sikic BI . Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol 1999; 17: 1061–70.
Wood KW, Cornwell WD, Jackson JR . Past and future of the mitotic spindle as an oncology target. Curr Opin Pharmacol 2001; 1: 370–7.
Sarli V, Giannis A . Inhibitors of mitotic kinesins: next-generation antimitotics. ChemMedChem 2006; 1: 293–8.
Romagnoli R, Baraldi PG, Carrion MD, Lopez Cara C, Preti D, Fruttarolo F, et al. Synthesis and biological evaluation of 2- and 3-aminobenzo[b]thiophene derivatives as antimitotic agents and inhibitors of tubulin polymerization. J Med Chem 2007; 50: 2273–7.
Pellegrini F, Budman DR . Review: tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest 2005; 23: 264–73.
Honore S, Pasquier E, Braguer D . Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci 2005; 62: 3039–56.
Chang JY, Hsieh HP, Chang CY, Hsu KS, Chiang YF, Chen CM, et al. 7-Aroyl-aminoindoline-1-sulfonamides as a novel class of potent antitubulin agents. J Med Chem 2006; 49: 6656–9.
Nam NH . Combretastatin A-4 analogues as antimitotic antitumor agents. Curr Med Chem 2003; 10: 1697–722.
Hsieh HP, Liou JP, Mahindroo N . Pharmaceutical design of antimitotic agents based on combretastatins. Curr Pharm Des 2005; 11: 1655–77.
Tron GC, Pagliai F, Del Grosso E, Genazzani AA, Sorba G . Synthesis and cytotoxic evaluation of combretafurazans. J Med Chem 2005; 48: 3260–8.
Das U, Gul HI, Alcorn J, Shrivastav A, George T, Sharma RK, et al. Cytotoxic 5-aryl-1-(4-nitrophenyl)-3-oxo-1,4-pentadienes mounted on alicyclic scaffolds. Eur J Med Chem 2006; 41: 577–85.
Hu L, Jiang JD, Qu J, Li Y, Jin J, Li ZR, et al. Novel potent antimitotic heterocyclic ketones: synthesis, antiproliferative activity, and structure-activity relationships. Bioorg Med Chem Lett 2007; 17: 3613–7.
Liu ZY, Wang YM, Li ZR, Jiang JD, Boykin DW . Synthesis and anticancer activity of novel 3,4-diarylthiazol-2(3H)-ones(imines). Bioorg Med Chem Lett 2009; 19: 5661–4.
John S, Thangapandian S, Arooj M, Hong JC, Kim KD, Lee KW . Development, evaluation and application of 3D QSAR pharmacophore model in the discovery of potential human renin inhibitors. BMC Bioinformatics 2011; 12 Suppl 14: S4.
Arooj M, Thangapandian S, John S, Hwang S, Park JK, Lee KW . 3D QSAR pharmacophore modeling, in silico screening, and density functional theory (DFT) approaches for identification of human chymase inhibitors. Int J Mol Sci 2011; 12: 9236–64.
John S, Thangapandian S, Sakkiah S, Lee KW . Potent BACE-1 inhibitor design using pharmacophore modeling, in silico screening and molecular docking studies. BMC Bioinformatics 2011; 12 Suppl 1: S28.
Vitorović-Todorović MD, Erić-Nikolić A, Kolundžija B, Hamel E, Ristić S, Juranić IO, et al. (E)-4-aryl-4-oxo-2-butenoic acid amides, chalcone-aroylacrylic acid chimeras: design, antiproliferative activity and inhibition of tubulin polymerization. Eur J Med Chem 2013; 62: 40–50.
Prinz H, Schmidt P, Böhm KJ, Baasner S, Müller K, Gerlach M, et al. Phenylimino-10H-anthracen-9-ones as novel antimicrotubule agents-synthesis, antiproliferative activity and inhibition of tubulin polymerization. Bioorg Med Chem 2011; 19: 4183–91.
Krishnegowda G, Prakasha Gowda AS, Tagaram HR, Carroll KF, Irby RB, Sharma AK, et al. Synthesis and biological evaluation of a novel class of isatin analogs as dual inhibitors of tubulin polymerization and Akt pathway. Bioorg Med Chem 2011; 19: 6006–14.
Carta A, Briguglio I, Piras S, Boatto G, La Colla P, Loddo R, et al. 3-Aryl-2-[1H-benzotriazol-1-yl]acrylonitriles: a novel class of potent tubulin inhibitors. Eur J Med Chem 2011; 46: 4151–67.
Kamal A, Reddy MK, Shaik TB, Rajender, Srikanth YV, Reddy VS, et al. Synthesis of terphenyl benzimidazoles as tubulin polymerization inhibitors. Eur J Med Chem 2012; 50: 9–17.
Vitorović-Todorović MD, Erić-Nikolić A, Kolundžija B, Hamel E, Ristić S, Juranić IO, et al. (E)-4-aryl-4-oxo-2-butenoic acid amides, chalcone-aroylacrylic acid chimeras: design, antiproliferative activity and inhibition of tubulin polymerization. Eur J Med Chem 2013; 62: 40–50.
Wang XF, Wang SB, Ohkoshi E, Wang LT, Hamel E, Qian K, et al. N-aryl-6-methoxy-1,2,3,4-tetrahydroquinolines: a novel class of antitumor agents targeting the colchicine site on tubulin. Eur J Med Chem 2013; 67: 196–207.
Monk KA, Siles R, Hadimani MB, Mugabe BE, Ackley JF, Studerus SW, et al. Design, synthesis, and biological evaluation of combretastatin nitrogen-containing derivatives as inhibitors of tubulin assembly and vascular disrupting agents. Bioorg Med Chem 2006; 14: 3231–44.
Wang XF, Ohkoshi E, Wang SB, Hamel E, Bastow KF, Morris-Natschke SL, et al. Synthesis and biological evaluation of N-alkyl-N-(4-methoxyphenyl)pyridin-2-amines as a new class of tubulin polymerization inhibitors. Bioorg Med Chem 2013; 21: 632–42.
Wang G, Peng F, Cao D, Yang Z, Han X, Liu J, et al. Design, synthesis and biological evaluation of millepachine derivatives as a new class of tubulin polymerization inhibitors. Bioorg Med Chem 2013; 21: 6844–54.
Nakamura M, Kajita D, Matsumoto Y, Hashimoto Y . Design and synthesis of silicon-containing tubulin polymerization inhibitors: replacement of the ethylene moiety of combretastatin A-4 with a silicon linker. Bioorg Med Chem 2013; 21: 7381–91.
Stoll F, Liesener S, Hohlfeld T, Schrör K, Fuchs PL, Höltje HD . Pharmacophore definition and three-dimensional quantitative structure-activity relationship study on structurally diverse prostacyclin receptor agonists. Mol Pharmacol 2002; 62: 1103–11.
Zampieri D, Mamolo MG, Laurini E, Florio C, Zanette C, Fermeglia M, et al. Synthesis, biological evaluation, and three-dimensional in silico pharmacophore model for sigma(1) receptor ligands based on a series of substituted benzo[d]oxazol-2(3H)-one derivatives. J Med Chem 2009; 52: 5380–93.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ . Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001; 46: 3–26.
Dearden JC . In silico prediction of drug toxicity. J Comput Aided Mol Des 2003; 17: 119–27.
Sutherland JJ, Raymond JW, Stevens JL, Baker TK, Watson DE . Relating molecular properties and in vitro assay results to in vivo drug disposition and toxicity outcomes. J Med Chem 2012; 55: 6455–66.
Labute P . Protonate3D: assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 2009; 75: 187–205.
Goto J, Kataoka R, Muta H, Hirayama N . ASEDock-docking based on alpha spheres and excluded volumes. J Chem Inf Model 2008; 48: 583–90.
Ducki S1, Rennison D, Woo M, Kendall A, Chabert JF, McGown AT, et al. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: synthesis and biological evaluation of antivascular activity. Bioorg Med Chem 2009; 17: 7698–710.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No 81220108012, 61335007, 81371684, 81000666, 81171395 and 81328012).
Author information
Authors and Affiliations
Corresponding author
Additional information
The supplementary figure was available on the APS's website.
Supplementary information
Supplementary information, Figure S1
Supplementary information (JPG 546 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Niu, Mm., Qin, Jy., Tian, Cp. et al. Tubulin inhibitors: pharmacophore modeling, virtual screening and molecular docking. Acta Pharmacol Sin 35, 967–979 (2014). https://doi.org/10.1038/aps.2014.34
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.34
Keywords
This article is cited by
-
New antiproliferative 3-substituted oxindoles inhibiting EGFR/VEGFR-2 and tubulin polymerization
Molecular Diversity (2024)
-
Cellular, Biophysical and in Silico Binding Study of β-Estradiol-6-one 6- (O-carboxy methyl Oxime) with Tubulin in Search of Antimitotic Derivative of 2-Methoxy Estradiol
Cell Biochemistry and Biophysics (2023)
-
Geo-environmental factors and the effectiveness of mulberry leaf extract in managing malaria
Scientific Reports (2023)
-
Discriminating agonist and antagonist ligands of the nuclear receptors using 3D-pharmacophores
Journal of Cheminformatics (2016)
-
Novel chemical scaffolds of the tumor marker AKR1B10 inhibitors discovered by 3D QSAR pharmacophore modeling
Acta Pharmacologica Sinica (2015)