Abstract
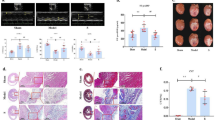
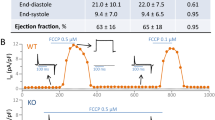
Both iptakalim (Ipt) and natakalim (Nat) activate the SUR2B/Kir6.1 channel, an ATP-sensitive potassium channel (KATP) subtype, with high selectivity. In this study we investigated the therapeutic effects of Ipt and Nat against isoproterenol-induced chronic heart failure (ISO-CHF) in rats, and demonstrated a new therapeutic approach to the treatment of CHF through activation of the SUR2B/Kir6.1 channel in endothelial cells. In ISO-CHF rats, oral administration of Nat (1, 3, 9 mg·kg−1·d−1) or Ipt (3 mg·kg−1·d−1) for 60 days significantly improved cardiac dysfunction, reversed cardiac remodeling, significantly attenuated the pathological increases in BNP levels, and improved endothelial dysfunction by adjusting the balance between endothelin and NO systems. The therapeutic effects of Nat were prevented by the selective KATP blocker glibenclamine (Gli, 50 mg·kg−1·d−1), confirming that these effects were mediated through activation of the SUR2B/Kir6.1 channel in endothelial cells. The molecular mechanisms underlying the therapeutic effects of Nat were further addressed using proteomic methods. We identified 724 proteins in the plasma of ISO-CHF rats; 55 proteins were related to Nat. These differentially expressed proteins were mainly involved in single-organism processes and the regulation of biological quality relative to CHF, including proteasome (Psm) and ATP protein clusters. We screened out PRKAR2β, GAS6/eNOS/NO and NO/PKG/VASP pathways involved in the amelioration of CHF among the 24 enriched pathways. We further confirmed 6 protein candidates, including PRKAR2β, GAS6 and VASP, which were involved in the endothelial mechanisms, and ATP, TIMP3 and AGT, which contributed to its cardiovascular actions. This study demonstrates a new pharmacological approach to the treatment of CHF through activation of the SUR2B/Kir6.1 channel in endothelial cells, and that the eNOS/VASP pathways are involved in its signaling mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jessup M, Brozena S . Heart failure. N Engl J Med 2003; 348: 2007–18.
Ryan PM, Lawrence C, Shah PK . Chronic heart failure. Am J Cardiovas Drugs 2011; 11: 153–71.
Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 1991; 325: 1468–75.
Farquharson CA1, Butler R, Hill A, Belch JJ, Struthers AD . Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002; 106: 221–6.
Tang X, Luo YX, Chen HZ, Liu DP . Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 2014; 5: 175.
Wang H, Long CL, Duan ZB, Shi CG, Jia GD, Zhang YL . A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res 2007; 73: 497–503.
Gao S, Long CL, Wang RH, Wang H . KATP activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res 2009; 83: 444–56.
Tang Y, Long CL, Wang RH, Cui WY, Wang H . Activation of SUR2B/Kir6.1 subtype of adenosine triphosphate-sensitive potassium channel improves pressure overload-induced cardiac remodeling via protecting endothelial function. J Cardiovasc Pharmacol 2010; 56: 345–53.
Zhou HM, Zhong ML, Zhang YF, Cui WY, Long CL, Wang H . Natakalim improves post-infarction left ventricular remodeling by restoring the coordinated balance between endothelial function and cardiac hypertrophy. Inter J Mol Med 2014; 34: 1209–18.
Zhong ML, Zhou HM, Long CL, Zhang YF, Cui WY, Wang H . Natakalim ameliorates isoproterenol-induced chronic heart failure by protecting against endothelial dysfunction. Pharmacology 2016; 98: 99–110.
Stéphanie FB, William CB, Adam AD, David ES, Peter CC, Joseph DT, et al. Plasma membrane proteomes of differentially matured dendritic cells identified by LC-MS/MS combined with iTRAQ labelling. J Proteomics 2012; 75: 938–48.
James DB, John PR, Arunangshu D, Jason L, Todd MU, Anne S, et al. Proteomic profiling of human plasma by iTRAQ reveals down-regulation of ITI-HC3 and VDBP by cigarette smoking. J Proteome Res 2011; 10: 1151–9.
Uros R, Kjell P, Jaco CK, Maarten L, Se bastien B, Oleg K, et al. iTRAQ-based proteomics profiling reveals increased metabolic activity and cellular cross-talk in angiogenic compared with invasive glioblastoma phenotype. Mol Cell Proteomics 2009; 8: 2595–612.
Stephan D, Winkler M, Kühner P, Russ U, Quast U . Selectivity of repaglinide and glibenclamide for the pancreatic over the cardiovascular K(ATP) channels. Diabetologia 2006; 49: 2039–48.
Seino S, Miki T . Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 2003; 81: 133–76.
Wang SY, Cui WY, Wang H . The new antihypertensive drug iptakalim activates ATP-sensitive potassium channels in the endothelium of resistance blood vessels. Acta Pharmacol Sin 2015; 36: 1444–50.
Scherrer CM, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, et al. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 2001; 104: 1286–91.
Kobayashi N, Mori Y, Nakano S, Tsubokou Y, Shirataki H, Matsuoka H . Celiprolol stimulates endothelial nitric oxide synthase expression and improves myocardial remodeling in deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 2001; 19: 795–801.
Bras-Silva C, Castro-Chaves PM, Fontes-Sousa AP, Nunes P, Monteiro-Sousa D, Duarte AJ, et al. Impaired systolic and diastolic myocardial response to ET-1 and Ang II in heart failure. Eur J Heart Fail 2006; 5: S56–7.
Liu TT, Le CN, Kime EJ, Trand D, Phinney BS, Anne AK . Mitochondrial proteome remodeling in ischemic heart failure. Life Sci 2014; 101: 27–36.
Planavila A, Redondo AI, Ribas F, Garrabou G, Casademont J, Giralt M, et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 2015; 106: 1–13.
Tuunanen H, Knuuti J . Metabolic remodeling in human heart failure. Cardiovasc Res 2011; 90: 251–7.
Chen XJ, Han WZ, Zhang YF, Cui WY, Pan ZY, Long CL, et al. The molecular pathway of ATP-sensitive potassium channel in endothelial cells for mediating arteriole relaxation. Life Sci 2015; 137: 164–9.
Zhao Fl, Fu L, Yang W, Dong YH, Yang J, Sun SB . Cardioprotective effects of baicalein on heart failure via modulation of Ca2+ handling proteins in vivo and in vitro. Life Sci 2016; 145: 213–23.
Gao M, Wang Y, Wang H . Effects of iptakalim on intracellular calcium concentrations, PKA and PKC activities in rat tail artery smooth muscle cells. Acta Pharmacol Sin 2005; 40: 954–7.
Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR . Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A 2006; 103: 3511–8.
Hasanbasic I, Cuerquis J, Varnum B, Blostein MD . Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 2004; 287: H1207–H1213.
Laurance S, Aghourain MN, Lila ZJ, Lemarie CA, Blostein MD . Gas6-induced tissue factor expression in endothelial cells is mediated through caveolin-1-enriched microdomains. J Thromb Haemost 2014; 12: 395–408.
Son BK, Kozaki K, Iijima K, Eto M, Nakano T, Akishita M, et al. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol 2007; 556: 1–8.
Wang B, Yang Q, Bai WW, Xing YF, Lu XT, Sun YY, et al. Tongxinluo protects against pressure overload-induced heart failure in mice involving VEGF/Akt/eNOS pathway activation. PloS One 2014; 9: e98047.
Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, et al. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol 2007; 179: 761–75.
Ibarra-Alvarado C, Galle J, Melichar VO, Mameghani A, Schmidt HH . Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol Pharmacol 2002; 61: 312–9.
Schmit MA, Mirakaj V, Stangassinger M, König K, Köhler D, Rosenberger P . Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation 2012; 35: 566–73.
Lindsey ML, Zamilpa R . Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 2012; 30: 31–41.
Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, et al. Combination of tumor necrosis factor-α ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res 2005; 97: 38090.
Moria J, Zhang LY, Oudit GY, Lopaschuk GD . Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure. J Mol Cell Cardiol 2013; 63: 98–106.
Acknowledgements
This work was supported by grants from the National Basic Research “973” Program (Grants No 2012CB518200 and JCKY2013000B001) and the State Key Research Project of China (Grant No AWS11J003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information is available on Acta Pharmacologica Sinica's web site.
Supplementary information
Supplementary Figure S1
Gene Ontology (GO) functional annotations of all proteins. (DOC 103 kb)
Supplementary Figure S2
Cluster of Orthologous Groups (COG) of all proteins. (DOC 82 kb)
Supplementary Table S1
The differential proteins in the plasma of model and Nat 3mg/kg treated group from three independent biological replicate experiments (DOC 90 kb)
Supplementary Table S2
The differential proteins in the plasma of control and model group from three independent biological replicate experiments (DOC 143 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Long, Cl., Chen, J. et al. Pharmacological evidence: a new therapeutic approach to the treatment of chronic heart failure through SUR2B/Kir6.1 channel in endothelial cells. Acta Pharmacol Sin 38, 41–55 (2017). https://doi.org/10.1038/aps.2016.118
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.118
Keywords
This article is cited by
-
The impact of oral anti-diabetic medications on heart failure: lessons learned from preclinical studies
Heart Failure Reviews (2018)