Abstract
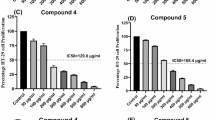
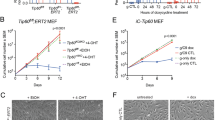
Histone deacetylases (HDACs), especially HDAC1, 2, 3 and 4, are abundantly expressed and over-activated in prostate cancer that is correlated with the poor prognosis. Thus, inhibition of HDAC activity has emerged as a potential alternative option for prostate cancer therapy. Chromopeptide A is a depsipeptide isolated from the marine sediment-derived bacterium Chromobacterium sp. HS-13-94; it has a chemical structure highly similar to FK228, a class I HDAC inhibitor that is approved by FDA for treating T-cell lymphoma. In this study, we determined whether chromopeptide A, like FK228, acted as a class I HDAC inhibitor, and whether chromopeptide A could inhibit the growth and migration of human prostate cancer in vitro and in vivo. HDAC enzyme selectivity and kinetic analysis revealed that chromopeptide A selectively inhibited the enzymatic activities of HDAC1, 2, 3 and 8 in a substrate non-competitive manner with comparable IC50 values for each HDAC member as FK228 in vitro. Importantly, chromopeptide A dose-dependently suppressed the proliferation of human prostate cancer cell lines PC3, DU145 and LNCaP with IC50 values of 2.43±0.02, 2.08±0.16, and 1.75±0.06 nmol/L, respectively, accompanied by dose-dependent inhibition of HDAC enzymatic activity in PC3 and DU145 cells. Chromopeptide A (0.2–50 nmol/L) caused G2/M phase arrest and induced apoptosis in the prostate cancer cell lines. Moreover, chromopeptide A dose-dependently inhibited the migration of PC3 cells. In mice bearing PC3 prostate cancer xenografts, intravenous injection of chromopeptide A (1.6, 3.2 mg/kg, once a week for 18 d) significantly suppressed the tumor growth, which was associated with increased expression levels of Ac-H3 and p21 in tumor tissues. Our results identify chromopeptide A as a novel class I HDAC inhibitor and provide therapeutic strategies that may be implemented in prostate cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2016. Ca-Cancer J Clin 2016; 66: 7–30.
Sutcliffe S, Colditz GA . Prostate cancer: is it time to expand the research focus to early-life exposures? Nat Rev Cancer 2013; 13: 208–18.
Sharifi N, Gulley JL, Dahut WL . Androgen deprivation therapy for prostate cancer. JAMA 2005; 294: 238–44.
Yap TA, Smith AD, Ferraldeschi R, Al-Lazikani B, Workman P, de Bono JS . Drug discovery in advanced prostate cancer: translating biology into therapy. Nat Rev Drug Discov 2016; 15: 699–718.
Karantanos T, Corn PG, Thompson TC . Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013; 32: 5501–11.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2011; 59: 572–83.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12.
Berthold DR, Pond GR, de Wit R, Eisenberger M, Tannock IF . Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. Ann Oncol 2008; 19: 1749–53.
Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther 2013; 12: 1629–37.
Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 2011; 17: 5913–25.
Waltregny D, North B, Van Mellaert F, de Leval J, Verdin E, Castronovo V . Screening of histone deacetylases (HDAC) expression in human prostate cancer reveals distinct class I HDAC profiles between epithelial and stromal cells. Eur J Histochem 2004; 48: 273–90.
Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN . Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate 2004; 59: 177–89.
Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 2008; 98: 604–10.
Li LH, Zhang PR, Cai PY, Li ZC . Histone deacetylase inhibitor, Romidepsin (FK228) inhibits endometrial cancer cell growth through augmentation of p53-p21 pathway. Biomed Pharmacother 2016; 82: 161–6.
Ruscetti M, Dadashian EL, Guo W, Quach B, Mulholland DJ, Park JW, et al. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 2016; 35: 3781–95.
Munster PN, Marchion D, Thomas S, Egorin M, Minton S, Springett G, et al. Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br J Cancer 2009; 101: 1044–50.
Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the department of defense prostate cancer clinical trial consortium and university of chicago phase 2 consortium. Cancer 2009; 115: 5541–9.
Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S, et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 2010; 21: 109–13.
Duran N, Justo GZ, Ferreira CV, Melo PS, Cordi L, Martins D . Violacein: properties and biological activities. Biotechnol Appl Biochem 2007; 48: 127–33.
VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH . Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot (Tokyo) 2011; 64: 525–31.
Zhou Z, Wang X, Zhang H, Sun J, Zheng L, Liu H, et al. Chromopeptide A, a highly cytotoxic depsipeptide from the marine sediment-derived bacterium Chromobacterium sp. HS-13-94. Acta Pharm Sin B 2015; 5: 62–6.
Ocker M, Schneider-Stock R . Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol 2007; 39: 1367–74.
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA . Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A 2004; 101: 1241–6.
Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51.
Abbas A, Gupta S . The role of histone deacetylases in prostate cancer. Epigenetics 2008; 3: 300–9.
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK . Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001; 1: 194–202.
Lane AA, Chabner BA . Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 2009; 27: 5459–68.
Suresh PS, Devaraj VC, Srinivas NR, Mullangi R . Review of bioanalytical assays for the quantitation of various HDAC inhibitors such as vorinostat, belinostat, panobinostat, romidepsin and chidamine. Biomed Chromatogr 2017; 31. doi: 10.1002/bmc.3807.
Kaushik D, Vashistha V, Isharwal S, Sediqe SA, Lin MF . Histone deacetylase inhibitors in castration-resistant prostate cancer: molecular mechanism of action and recent clinical trials. Ther Adv Urol 2015; 7: 388–95.
Liu Z, Ding K, Li L, Liu H, Wang Y, Liu C, et al. A novel histone deacetylase inhibitor Chidamide induces G0/G1 arrest and apoptosis in myelodysplastic syndromes. Biomed Pharmacother 2016; 83: 1032–7.
Xue K, Gu JJ, Zhang Q, Mavis C, Hernandez-Ilizaliturri FJ, Czuczman MS, et al. Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents. J Cancer Res Clin Oncol 2016; 142: 379–87.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81673472, No 81402966 to Ai-jun SHEN and 81520108028 to Yue-wei GUO); the National Program on Key Basic Research Project of China (No 2012CB910704 to Mei-yu GENG); and the Personalized Medicines-Molecular Signature-Based Drug Discovery and Development, Strategic Priority Research Program of the Chinese Academy of Sciences (No XDA12020105 to Ai-jun SHEN).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary Figure 1
Chromopeptide A induces apoptosis through caspase 9 dependent intrinsic pathway in prostate cancer cells. (JPG 674 kb)
Rights and permissions
About this article
Cite this article
Sun, Jy., Wang, Jd., Wang, X. et al. Marine-derived chromopeptide A, a novel class I HDAC inhibitor, suppresses human prostate cancer cell proliferation and migration. Acta Pharmacol Sin 38, 551–560 (2017). https://doi.org/10.1038/aps.2016.139
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.139
Keywords
This article is cited by
-
FK228 reshapes tumor microenvironment to enhance anti-PD-L1 efficacy
Oncogene (2025)
-
Marine life as a source of anti-prostate cancer agents: an updated overview (2003–2023)
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Bacterial Peptides and Bacteriocins as Novel Treatment for Prostate Cancer
International Journal of Peptide Research and Therapeutics (2023)
-
Potential Role of Herbal- and Bacterial-Derived Peptides Against Colorectal Cancer
Revista Brasileira de Farmacognosia (2022)
-
USP38 regulates the stemness and chemoresistance of human colorectal cancer via regulation of HDAC3
Oncogenesis (2020)