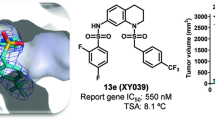
Abstract
Aim:
Retinoic acid receptor-related orphan nuclear receptors (RORs) are orphan nuclear receptors that show constitutive activity in the absence of ligands. Among 3 subtypes of RORs, RORc is a promising therapeutic target for the treatment of Th17-mediated autoimmune diseases. Here, we report novel RORc inverse agonists discovered through structure-based drug design.
Methods:
Based on the structure of compound 8, a previously described agonist of RORa, a series of 4-(4-(benzyloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-one derivatives were designed and synthesized. The interaction between the compounds and RORc was detected at molecular level using AlphaScreen assay. The compounds were further examined in 293T cells transfected with RORc and luciferase reporter gene. Thermal stability shift assay was used to evaluate the effects of the compounds on protein stability.
Results:
A total of 27 derivatives were designed and synthesized. Among them, the compound 22b was identified as the most potent RORc inverse agonist. Its IC50 values were 2.39 μmol/L in AlphaScreen assay, and 0.82 μmol/L in inhibition of the cell-based luciferase reporter activity. Furthermore, the compound 22b displayed a 120-fold selectivity for RORc over other nuclear receptors. Moreover, a molecular docking study showed that the structure-activity relationship was consistent with the binding mode of compound 22b in RORc.
Conclusion:
4-(4-(Benzyloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-one derivatives are promising candidates for the treatment of Th17-mediated autoimmune diseases, such as rheumatoid arthritis, psoriasis, and multiple sclerosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Kamenecka TM, Lyda B, Chang MR, Griffin PR . Synthetic modulators of the retinoic acid receptor-related orphan receptors. Med Chem Comm 2013; 4: 764–76.
Toyama H, Nakamura M, Nakamura M, Matsumoto Y, Nakagomi M, Hashimoto Y . Development of novel silicon-containing inverse agonists of retinoic acid receptor-related orphan receptors. Bioorg Med Chem 2014; 22: 1948–59.
Wang Y, Cai W, Zhang G, Yang T, Liu Q, Cheng Y, et al. Discovery of novel N-(5-(arylcarbonyl)thiazol-2-yl)amides and N-(5-(arylcarbonyl)thiophen-2-yl)amides as potent RORgammat inhibitors. Bioorg Med Chem 2014; 22: 692–702.
Jetten AM, Ueda E . Retinoid-related orphan receptors (RORs): roles in cell survival, differentiation and disease. Cell Death Differ 2002; 9: 1167–71.
Gege C, Schluter T, Hoffmann T . Identification of the first inverse agonist of retinoid-related orphan receptor (ROR) with dual selectivity for RORbeta and RORgammat. Bioorg Med Chem Lett 2014; 24: 5265–7.
Chao J, Enyedy I, Van Vloten K, Marcotte D, Guertin K, Hutchings R, et al. Discovery of biaryl carboxylamides as potent RORgamma inverse agonists. Bioorg Med Chem Lett 2015; 25: 2991–7.
Ranhotra HS . The interplay between retinoic acid receptor-related orphan receptors and human diseases. J Recept Signal Transduct Res 2012; 32: 181–9.
Solt LA, Griffin PR, Burris TP . Ligand regulation of retinoic acid receptor-related orphan receptors: implications for development of novel therapeutics. Curr Opin Lipidol 2010; 21: 204–11.
Khan PM, El-Gendy Bel D, Kumar N, Garcia-Ordonez R, Lin L, Ruiz CH, et al. Small molecule amides as potent ROR-gamma selective modulators. Bioorg Med Chem Lett 2013; 23: 532–6.
Ouyang W, Kolls JK, Zheng Y . The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28: 454–67.
Zhang Y, Xue X, Jin X, Song Y, Li J, Luo X, et al. Discovery of 2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamide derivatives as new RORgamma inhibitors using virtual screening, synthesis and biological evaluation. Eur J Med Chem 2014; 78: 431–41.
Fauber BP, Magnuson S . Modulators of the nuclear receptor retinoic acid receptor-related orphan receptor-gamma (RORgamma or RORc). J Med Chem 2014; 57: 5871–92.
Huh JR, Littman DR . Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol 2012; 42: 2232–7.
Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem 2011; 286: 31409–17.
Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 2011; 472: 486–90.
Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C . Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem 2011; 286: 22707–10.
Kumar N, Lyda B, Chang MR, Lauer JL, Solt LA, Burris TP, et al. Identification of SR2211: a potent synthetic RORgamma-selective modulator. ACS Chem Biol 2012; 7: 672–7.
Solt LA, Kumar N, He Y, Kamenecka TM, Griffin PR, Burris TP . Identification of a selective RORgamma ligand that suppresses T(H)17 cells and stimulates T regulatory cells. ACS Chem Biol 2012; 7: 1515–9.
Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 2011; 472: 491–4.
Fauber BP, Rene O, de Leon Boenig G, Burton B, Deng Y, Eidenschenk C, et al. Reduction in lipophilicity improved the solubility, plasma-protein binding, and permeability of tertiary sulfonamide RORc inverse agonists. Bioorg Med Chem Lett 2014; 24: 3891–7.
Fauber BP, de Leon Boenig G, Burton B, Eidenschenk C, Everett C, Gobbi A, et al. Structure-based design of substituted hexafluoroisopropanol-arylsulfonamides as modulators of RORc. Bioorg Med Chem Lett 2013; 23: 6604–9.
Yang T, Liu Q, Cheng Y, Cai W, Ma Y, Yang L, et al. Discovery of tertiary amine and indole derivatives as potent RORgammat inverse agonists. ACS Med Chem Lett 2014; 5: 65–8.
Dubernet M, Duguet N, Colliandre L, Berini C, Helleboid S, Bourotte M, et al. Identification of new nonsteroidal RORalpha ligands; related structure-activity relationships and docking studies. ACS Med Chem Lett 2013; 4: 504–8.
Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y . Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol 2010; 24: 923–9.
Suino-Powell K, Xu Y, Zhang C, Tao YG, Tolbert WD, Simons SS Jr, et al. Doubling the size of the glucocorticoid receptor ligand binding pocket by deacylcortivazol. Mol Cell Biol 2008; 28: 1915–23.
Niesen FH, Berglund H, Vedadi M . The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2007; 2: 2212–21.
Pantoliano MW, Petrella EC, Kwasnoski JD, Lobanov VS, Myslik J, Graf E, et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen 2001; 6: 429–40.
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 2004; 47: 1739–49.
Li Z, Wang X, Xu X, Yang J, Xia W, Zhou X, et al. Design, synthesis and biological activity of phenoxyacetic acid derivatives as novel free fatty acid receptor 1 agonists. Bioorg Med Chem 2015; 23: 7158–64.
van Niel MB, Fauber BP, Cartwright M, Gaines S, Killen JC, Rene O, et al. A reversed sulfonamide series of selective RORc inverse agonists. Bioorg Med Chem Lett 2014; 24: 5769–76.
Acknowledgements
We gratefully acknowledge financial support partly from the National Natural Science Foundation of China (81373325), the “100 Talents Project” of Chinese Academy of Sciences, the Natural Science Foundation of Guangdong Province (c15140600000016), the Guangzhou Healthcare Collaborative Innovation Programs (201508020255), the Chinese Academy of Sciences Cloud Platform for Stem Cell and Biomedical Research (XXH12503-05-06), the National Key Basic Research Program of China (973 Program, 2013CB910601) and Bureau of Science and Information Technology of Guangzhou Municipality (2013J4500008). The authors gratefully acknowledge support from the Guangzhou Branch of the Supercomputing Center of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available at the Acta Pharmacologica Sinica's website.
Supplementary information
Supplementary information
Supplementary information (PDF 429 kb)
Rights and permissions
About this article
Cite this article
Wu, Xs., Wang, R., Xing, Yl. et al. Discovery and structural optimization of 4-(4-(benzyloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-ones as RORc inverse agonists. Acta Pharmacol Sin 37, 1516–1524 (2016). https://doi.org/10.1038/aps.2016.32
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.32
Keywords
This article is cited by
-
Molecular dynamics simulations on RORγt: insights into its functional agonism and inverse agonism
Acta Pharmacologica Sinica (2019)