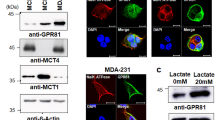
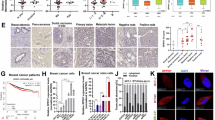
Abstract
The G protein-coupled receptor 55 (GPR55) is expressed in multiple tissues, and has been implicated in cancer pathogenesis, but little is known about its role in the migratory behavior of cancer cells, particularly breast cancer cells. In this study we first showed that GPR55 expression levels in 38 metastatic lymph nodes of breast cancer patients were profoundly elevated, and were positively associated in human breast cancer cells with their migratory ability. Moreover, the plasma levels of GPR55 endogenous agonist L-a-lysophosphatidylinositol (LPI) were significantly increased in breast cancer patients compared with healthy individuals. In human breast cancer LM-MCF-7 and MDA-MB-231 cells, treatment with LPI (2.5 μmol/L) significantly increased filopodia formation and resulted in cell migration, which could be blocked either by the GPR55 antagonist CID16020046 or by siRNA-mediated GPR55 knockdown. Furthermore, dual-luciferase report gene assays showed that GPR55 upregulated HBXIP at the promoter; GPR55 expression levels were positively correlated with HBXIP expression levels in breast cancer tissues and 8 breast cancer cell lines. We also showed that the LPI/GPR55 axis promoted the migration of breast cancer cells via two mutually exclusive pathways — the HBXIP/p-ERK1/2/Capn4 and MLCK/MLC signaling pathways. In xenograft nude mouse model, loss of GPR55 mainly affected breast cancer cell metastasis and the formation of metastatic foci. Thus, GPR55 is involved in the migratory behavior of human breast cancer cells and could serve as a pharmacological target for preventing metastasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Andradas C, Caffarel MM, Perez-Gomez E, Salazar M, Lorente M, Velasco G, et al. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 2011; 30: 245–52.
Ford LA, Roelofs AJ, Anavi-Goffer S, Mowat L, Simpson DG, Irving AJ, et al. A role for L-alpha-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br J Pharmacol 2010; 160: 762–71.
Pineiro R, Maffucci T, Falasca M . The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2011; 30: 142–52.
Perez-Gomez E, Andradas C, Flores JM, Quintanilla M, Paramio JM, Guzman M, et al. The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 2013; 32: 2534–42.
Henstridge CM, Balenga NA, Kargl J, Andradas C, Brown AJ, Irving A, et al. Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol 2011; 25: 1835–48.
Ross RA . L-alpha-lysophosphatidylinositol meets GPR55: a deadly relationship. Trends Pharmacol Sci 2011; 32: 265–9.
Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T . Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 2007; 362: 928–34.
Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K . GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 2008; 105: 2699–704.
Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A 2009; 106: 16511–6.
Oka S, Kimura S, Toshida T, Ota R, Yamashita A, Sugiura T . Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem 2010; 147: 671–8.
Moreno-Navarrete JM, Catalan V, Whyte L, Diaz-Arteaga A, Vazquez-Martinez R, Rotellar F, et al. The L-alpha-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 2012; 61: 281–91.
Xiao Y, Chen Y, Kennedy AW, Belinson J, Xu Y . Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses. Ann N Y Acad Sci 2000; 905: 242–59.
Shen Z, Wu M, Elson P, Kennedy AW, Belinson J, Casey G, et al. Fatty acid composition of lysophosphatidic acid and lysophosphatidylinositol in plasma from patients with ovarian cancer and other gynecological diseases. Gynecol Oncol 2001; 83: 25–30.
Falasca M, Silletta MG, Carvelli A, Di Francesco AL, Fusco A, Ramakrishna V, et al. Signalling pathways involved in the mitogenic action of lysophosphatidylinositol. Oncogene 1995; 10: 2113–24.
Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J 2003; 22: 2729–40.
Wen Y, Golubkov VS, Strongin AY, Jiang W, Reed JC . Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation. J Biol Chem 2008; 283: 2793–803.
Liu Q, Bai X, Li H, Zhang Y, Zhao Y, Zhang X, et al. The oncoprotein HBXIP upregulates Lin28B via activating TF II D to promote proliferation of breast cancer cells. Int J Cancer 2013; 133: 1310–22.
Xu F, You X, Liu F, Shen X, Yao Y, Ye L, et al. The oncoprotein HBXIP up-regulates Skp2 via activating transcription factor E2F1 to promote proliferation of breast cancer cells. Cancer Lett 2013; 333: 124–32.
Yue L, Li L, Liu F, Hu N, Zhang W, Bai X, et al. The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis 2013; 34: 927–35.
Liu S, Li L, Zhang Y, Zhang Y, Zhao Y, You X, et al. The oncoprotein HBXIP uses two pathways to up-regulate S100A4 in promotion of growth and migration of breast cancer cells. J Biol Chem 2012; 287: 30228–39.
You J, Mi D, Zhou X, Qiao L, Zhang H, Zhang X, et al. A positive feedback between activated extracellularly regulated kinase and cyclooxygenase/lipoxygenase maintains proliferation and migration of breast cancer cells. Endocrinology 2009; 150: 1607–17.
Zhang F, Wang Q, Ye L, Feng Y, Zhang X . Hepatitis B virus X protein upregulates expression of calpain small subunit 1 via nuclear factor-kappaB/p65 in hepatoma cells. J Med Virol 2010; 82: 920–8.
Kong GY, Zhang JP, Zhang S, Shan CL, Ye LH, Zhang XD . Hepatitis B virus X protein promotes hepatoma cell proliferation via upregulation of MEKK2. Acta Pharmacol Sin 2011; 32: 1173–80.
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D . Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 2008; 49: 1137–46.
Fauland A, Kofeler H, Trotzmuller M, Knopf A, Hartler J, Eberl A, et al. A comprehensive method for lipid profiling by liquid chromatographyion cyclotron resonance mass spectrometry. J Lipid Res 2011; 52: 2314–22.
Wang Y, Cui M, Cai X, Sun B, Liu F, Zhang X, et al. The oncoprotein HBXIP up-regulates SCG3 through modulating E2F1 and miR-509-3p in hepatoma cells. Cancer Lett 2014; 352: 169–78.
Zhou X, Liu Y, You J, Zhang H, Zhang X, Ye L . Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2. Cancer Lett 2008; 270: 312–27.
Nilufar S, Morrow AA, Lee JM, Perkins TJ . FiloDetect: automatic detection of filopodia from fluorescence microscopy images. BMC Syst Biol 2013; 7: 66.
Paul RK, Wnorowski A, Gonzalez-Mariscal I, Nayak SK, Pajak K, Moaddel R, et al. (R,R')-4'-methoxy-1-naphthylfenoterol targets GPR55-mediated ligand internalization and impairs cancer cell motility. Biochem Pharmacol 2014; 87: 547–61.
Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J . Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 2014; 94: 235–63.
Xue F, Janzen DM, Knecht DA . Contribution of filopodia to cell migration: A mechanical link between protrusion and contraction. Int J Cell Biol 2010; 2010: 507821.
Li Y, Zhang Z, Zhou X, Li L, Liu Q, Wang Z, et al. The oncoprotein HBXIP enhances migration of breast cancer cells through increasing filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer Lett 2014; 355: 288–96.
Zhou XL, Qin XR, Zhang XD, Ye LH . Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling. Acta Pharmacol Sin 2010; 31: 202–10.
Dorsam RT, Gutkind JS . G-protein-coupled receptors and cancer. Nat Rev Cancer 2007; 7: 79–94.
Kargl J, Andersen L, Hasenohrl C, Feuersinger D, Stancic A, Fauland A, et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br J Pharmacol 2016; 173: 142–54.
Hu N, Zhang J, Cui W, Kong G, Zhang S, Yue L, et al. miR-520b regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8. J Biol Chem 2011; 286: 13714–22.
Ridley AJ . Life at the leading edge. Cell 2011; 145: 1012–22.
Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, et al. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res 2004; 10: 4799–805.
Zheng PC, Chen X, Zhu HW, Zheng W, Mao LH, Lin C, et al. Capn4 is a marker of poor clinical outcomes and promotes nasopharyngeal carcinoma metastasis via nuclear factor-kappaB-induced matrix metalloproteinase 2 expression. Cancer Sci 2014; 105: 630–8.
Undyala VV, Dembo M, Cembrola K, Perrin BJ, Huttenlocher A, Elce JS, et al. The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration. J Cell Sci 2008; 121: 3581–8.
Wang GS, Huang YG, Li H, Bi SJ, Zhao JL . ERK/CANP rapid signaling mediates 17beta-estradiol-induced proliferation of human breast cancer cell line MCF-7 cells. Int J Clin Exp Med 2014; 7: 156–62.
Zhang X, You X, Wang Q, Zhang T, Du Y, Lv N, et al. Hepatitis B virus X protein drives multiple cross-talk cascade loops involving NF-kappaB, 5-LOX, OPN and Capn4 to promote cell migration. PLoS One 2012; 7: e31458.
Lai JM, Hsieh CL, Chang ZF . Caspase activation during phorbol ester-induced apoptosis requires ROCK-dependent myosin-mediated contraction. J Cell Sci 2003; 116: 3491–501.
Gutjahr MC, Rossy J, Niggli V . Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp Cell Res 2005; 308: 422–38.
Acknowledgements
This project was supported by grants from the National Natural Science Foundation of China (No 81602428 and 81402490) and the Colleges and Universities in Hebei Province Science and Technology Research Project (No QN2016020), and Science and Technology Research and Developmental Guidance Program of Shijiazhuang City (No 171201073A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary files are available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary information
Supplementary Table and Figure (DOC 83 kb)
Rights and permissions
About this article
Cite this article
Zhou, Xl., Guo, X., Song, Yp. et al. The LPI/GPR55 axis enhances human breast cancer cell migration via HBXIP and p-MLC signaling. Acta Pharmacol Sin 39, 459–471 (2018). https://doi.org/10.1038/aps.2017.157
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.157
Keywords
This article is cited by
-
Nanoinducer-mediated mitochondria-selective degradation enhances T cell immunotherapy against multiple cancers
Nature Nanotechnology (2025)
-
GPR55 senses lactate to sustain motility in prostate cancer cells
Molecular and Cellular Biochemistry (2025)
-
The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell’s metabolic crosstalk
BMC Medicine (2022)
-
The oncoprotein HBXIP competitively binds KEAP1 to activate NRF2 and enhance breast cancer cell growth and metastasis
Oncogene (2019)
-
The oncoprotein HBXIP promotes human breast cancer growth through down-regulating p53 via miR-18b/MDM2 and pAKT/MDM2 pathways
Acta Pharmacologica Sinica (2018)