Abstract
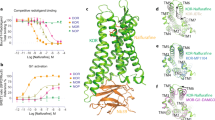
The μ opioid receptor (OR), a member of the class A subfamily of G-protein coupled receptors (GPCRs), is a major target for the treatment of pain. G-protein biased μ-OR agonists promise to be developed as analgesics. Thus, TRV130, the first representative μ-OR ligand with G-protein bias, has entered into phase III clinical trials. To identify the detailed G-protein-biased activation and inactivation mechanisms of the μ-OR, we constructed five μ-OR systems that were in complexes with the G-protein-biased agonists TRV130 and BU72, the antagonists β-FNA and naltrexone, as well as the free receptor. We performed a series of conventional molecular dynamics simulations and analyses of G-protein-biased activation and inactivation mechanisms of μ-OR. Our results, together with previously reported mutation results, revealed the operating mode of the activation switch composed of residues W6.48 and Y7.43 (Ballesteros/Weinstein numbering), the activity of which was responsible for down- and up-regulation, respectively, of the β-arrestin signaling, which in turn affected G-protein-biased activation of μ-OR. TRV130 was found to stabilize W6.48 by interacting with Y7.43. In addition, we obtained useful information regarding μ-OR-biased activation, such as strong stabilization of W7.35 through a hydrophobic ring interaction in the TRV130 system. These findings may facilitate understanding of μ-OR biased activation and the design of new biased ligands for GPCRs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lagerstrom MC, Schioth HB . Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 2008; 7: 339–57.
McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. β-Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–7.
Whalen EJ, Rajagopal S, Lefkowitz RJ . Therapeutic potential of β-arrestin and G protein-biased agonists. Trends Mol Med 2011; 17: 126–39.
Gurevich VV, Gurevich EV . The molecular acrobatics of arrestin activation. Trends Pharmacol Sci 2004; 25: 105–11.
Thomsen AR, Plouffe B, Cahill TJ, Shukla AK, Tarrasch JT, Dosey AM, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 2016; 166: 907–19.
Bruchas MR, Roth BL . New technologies for elucidating opioid receptor function. Trends Pharmacol Sci 2016; 37: 279–89.
Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG . Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 2000; 408: 720–3.
Raehal KM, Walker JK, Bohn LM . Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 2005; 314: 1195–201.
Thompson GL, Kelly E, Christopoulos A, Canals M . Novel GPCR paradigms at the mu-opioid receptor. Br J Pharmacol 2015; 172: 287–96.
Burford NT, Traynor JR, Alt A . Positive allosteric modulators of the mu-opioid receptor: a novel approach for future pain medications. Br J Pharmacol 2015; 172: 277–86.
Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem 2013; 56: 8019–31.
Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 2016; 157: 264–72.
Okude J, Ueda T, Kofuku Y, Sato M, Nobuyama N, Kondo K, et al. Identification of a conformational equilibrium that determines the efficacy and functional selectivity of the mu-opioid receptor. Angew Chem Int Ed Engl 2015; 54: 15771–6.
Schneider S, Provasi D, Filizola M . How oliceridine (TRV-130) binds and stabilizes a mu-opioid receptor conformational state that selectively triggers G protein signaling pathways. Biochemistry 2016; 55: 6456–66.
DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 2013; 344: 708–17.
Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014; 155: 1829–35.
Hothersall JD, Torella R, Humphreys S, Hooley M, Brown A, McMurray G, et al. Residues W320 and Y328 within the binding site of the mu-opioid receptor influence opiate ligand bias. Neuropharmacology 2017; 118: 46–58.
Ballesteros JA, Weinstein H . Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 1995; 25: 366–428.
Sun X, Laroche G, Wang X, Agren H, Bowman GR, Giguere PM, et al. Propagation of the allosteric modulation induced by sodium in the delta-opioid receptor. Chemistry 2017; 23: 4615–24.
Fenalti G, Giguere PM, Katritch V, Huang XP, Thompson AA, Cherezov V, et al. Molecular control of delta-opioid receptor signalling. Nature 2014; 506: 191–6.
Cheng J, Sun X, Li W, Liu G, Tu Y, Tang Y . Molecular switches of the kappa opioid receptor triggered by 6'-GNTI and 5'-GNTI. Sci Rep 2016; 6: 18913.
Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, et al. Structural insights into μ-opioid receptor activation. Nature 2015; 524: 315–21.
Neilan CL, Husbands SM, Breeden S, Ko MC, Aceto MD, Lewis JW, et al. Characterization of the complex morphinan derivative BU72 as a high efficacy, long-lasting mu-opioid receptor agonist. Eur J Pharmacol 2004; 499: 107–16.
Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 2012; 485: 321–6.
Spassov VZ, Flook PK, Yan L . LOOPER: a molecular mechanics-based algorithm for protein loop prediction. Protein Eng Des Sel 2008; 21: 91–100.
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 2004; 47: 1739–49.
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 2004; 47: 1750–9.
Lomize AL, Pogozheva ID, Mosberg HI . Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J Chem Inf Model 2011; 51: 930–46.
Lomize MA, Pogozheva ID, Joo H, Mosberg HI, Lomize AL . OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res 2012; 40: D370–6.
Klauda JB, Venable RM, Freites JA, O'Connor JW, Tobias DJ, Mondragon-Ramirez C, et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B 2010; 114: 7830–43.
Humphrey W, Dalke A, Schulten K . VMD: visual molecular dynamics. J Mol Graph 1996; 14: 33–8.
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 2010; 31: 671–90.
Vanommeslaeghe K . MacKerell AD Jr . Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J Chem Inf Model 2012; 52: 3144–54.
Vanommeslaeghe K, Raman EP . MacKerell AD Jr . Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J Chem Inf Model 2012; 52: 3155–68.
Hess B, Kutzner C, van der Spoel D, Lindahl E . GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 2008; 4: 435–47.
Chavkin C, Goldstein A . Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A 1981; 78: 6543–7.
Filizola M, Devi LA . Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci 2013; 34: 6–12.
Pasternak GW, Pan YX . Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 2013; 65: 1257–317.
Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, et al. Structure of the delta-opioid receptor bound to naltrindole. Nature 2012; 485: 400–4.
Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, Ernst OP . Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc Natl Acad Sci U S A 2003; 100: 2290–5.
Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S . Action of molecular switches in GPCRs--theoretical and experimental studies. Curr Med Chem 2012; 19: 1090–109.
Stenkamp RE, Teller DC, Palczewski K . Rhodopsin: a structural primer for G-protein coupled receptors. Arch Pharm (Weinheim) 2005; 338: 209–16.
Liu R, Nahon D, le Roy B, Lenselink EB, AP IJ . Scanning mutagenesis in a yeast system delineates the role of the NPxxY(x)(5,6)F motif and helix 8 of the adenosine A(2B) receptor in G protein coupling. Biochem Pharmacol 2015; 95: 290–300.
Sun X, Agren H, Tu Y . Functional water molecules in rhodopsin activation. J Phys Chem B 2014; 118: 10863–73.
Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016; 537: 185–90.
Shim J, Coop A . MacKerell AD Jr . Molecular details of the activation of the mu opioid receptor. J Phys Chem B 2013; 117: 7907–17.
Filizola M, Devi LA . Structural biology: how opioid drugs bind to receptors. Nature 2012; 485: 314–7.
Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012; 485: 327–32.
Acknowledgements
All simulations were performed on the high-performance computing cluster kindly provided by Prof Bang-ce YE of the State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology. This work was supported by the National Natural Science Foundation of China (Grants 81673356 and U1603122) and the 111 Project (Grant B07023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary files are available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary information
Supplementary Table and Figures (DOC 1357 kb)
Rights and permissions
About this article
Cite this article
Cheng, Jx., Cheng, T., Li, Wh. et al. Computational insights into the G-protein-biased activation and inactivation mechanisms of the μ opioid receptor. Acta Pharmacol Sin 39, 154–164 (2018). https://doi.org/10.1038/aps.2017.158
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.158
Keywords
This article is cited by
-
Encoding mu-opioid receptor biased agonism with interaction fingerprints
Journal of Computer-Aided Molecular Design (2021)