Abstract
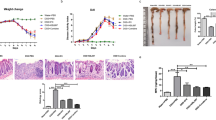
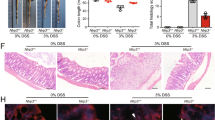
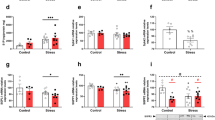
Osthole, a natural coumarin found in traditional Chinese medicinal plants, has shown multiple biological activities. In the present study, we investigated the preventive effects of osthole on inflammatory bowel disease (IBD). Colitis was induced in mice by infusing TNBS into the colonic lumen. Before TNBS treatment, the mice received osthole (100 mg·kg−1·d−1, ip) for 3 d. Pretreatment with osthole significantly ameliorated the clinical scores, colon length shortening, colonic histopathological changes and the expression of inflammatory mediators in TNBS-induced colitis. Pretreatment with osthole elevated serum cAMP levels; but treatment with the PKA inhibitor H89 (10 mg·kg−1·d−1, ip) did not abolish the beneficial effects of osthole on TNBS-induced colitis. In mouse peritoneal macrophages, pretreatment with osthole (50 μmol/L) significantly attenuated the LPS-induced elevation of cytokines at the mRNA level; inhibition of PKA completely reversed the inhibitory effects of osthole on IL-1β, IL-6, COX2, and MCP-1 but not on TNFα. In Raw264.7 cells, the p38 inhibitor SB203580 markedly suppressed LPS-induced upregulation of the cytokines, whereas the PKA inhibitors H89 or KT5720 did not abolish the inhibitory effects of SB203580. Moreover, in LPS-stimulated mouse peritoneal macrophages, SB203580 strongly inhibited the restored expression of IL-1β, IL-6, COX2, and MCP-1, which was achieved by abolishing the suppressive effects of osthole with the PKA inhibitors. Western blot analysis showed that osthole significantly suppressed the phosphorylation of p38, which was induced by TNBS in mice or by LPS in Raw264.7 cells. Inhibition of PKA partially reversed the suppressive effects of osthole on p38 phosphorylation in LPS-stimulated cells. Collectively, our results suggest that osthole is effective in the prevention of TNBS-induced colitis by reducing the expression of inflammatory mediators and attenuating p38 phosphorylation via both cAMP/PKA-dependent and independent pathways, among which the cAMP/PKA-independent pathway plays a major role.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Keighley MR, Stockbrugger RW . Inflammatory bowel disease. Aliment Pharmacol Ther 2003; 18: 66–70.
Tsujii M, Kawano S, Tsuji S, Kobayashi I, Takei Y, Nagano K, et al. Colonic mucosal hemodynamics and tissue oxygenation in patients with ulcerative colitis: investigation by organ reflectance spectrophotometry. J Gastroenterol 1995; 30: 183–8.
Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 2004; 286: G367–76.
Farrell RJ, Kelleher D . Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol 2003; 178: 339–46.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–76.
van Deventer SJ . Small therapeutic molecules for the treatment of inflammatory bowel disease. Gut 2002; 50: III47–53.
Torphy TJ . Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998; 157: 351–70.
Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, et al. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther 2000; 292: 22–30.
Loher F, Schmall K, Freytag P, Landauer N, Hallwachs R, Bauer C, et al. The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J Pharmacol Exp Ther 2003; 305: 549–56.
Rieder F, Siegmund B, Bundschuh DS, Lehr HA, Endres S, Eigler A . The selective phosphodiesterase 4 inhibitor roflumilast and phosphodiesterase 3/4 inhibitor pumafentrine reduce clinical score and TNF expression in experimental colitis in mice. PLoS One 2013; 8: e56867.
Sturgess I, Searle GF . The acute toxic effect of the phosphodiesterase inhibitor rolipram on plasma osmolality. Br J Clin Pharmacol 1990; 29: 369–70.
You L, Feng S, An R, Wang X . Osthole: a promising lead compound for drug discovery from a traditional Chinese medicine (TCM). Nat Prod Commun 2009; 4: 297–302.
Wang R, Kong J, Wang D, Lien LL, Lien EJ . A survey of Chinese herbal ingredients with liver protection activities. Chin Med 2007; 2: 5.
Hoult JR, Paya M . Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol 1996; 27: 713–22.
Chiu PR, Lee WT, Chu YT, Lee MS, Jong YJ, Hung CH . Effect of the Chinese herb extract osthol on IL-4-induced eotaxin expression in BEAS-2B cells. Pediatr Neonatol 2008; 49: 135–40.
Zimecki M, Artym J, Cisowski W, Mazol I, Wlodarczyk M, Glensk M . Immunomodulatory and anti-inflammatory activity of selected osthole derivatives. Z Naturforsch C 2009; 64: 361–8.
Liu J, Zhang W, Zhou L, Wang X, Lian Q . Anti-inflammatory effect and mechanism of osthole in rats. Zhong Yao Cai 2005; 28: 1002–6.
Matsuda H, Tomohiro N, Ido Y, Kubo M . Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol Pharm Bull 2002; 25: 809–12.
Zhang ZR, Leung WN, Cheung HY, Chan CW . Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid Based Complement Alternat Med 2015; 2015: 919616.
Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M . Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 2008; 39: 127–32.
Banner KH, Trevethick MA . PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci 2004; 25: 430–6.
Dang PM, Morel F, Gougerot-Pocidalo MA, El Benna J . Phosphorylation of the NADPH oxidase component p67(PHOX) by ERK2 and P38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry 2003; 42: 4520–6.
Tsai YF, Yu HP, Chung PJ, Leu YL, Kuo LM, Chen CY, et al. Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic Biol Med 2015; 89: 387–400.
Horie Y, Chiba M, Suzuki T, Kudo T, Kamata A, Iizuka M, et al. Induction of major histocompatibility complex class II antigens on human colonic epithelium by interferon-gamma, tumor necrosis factor-alpha, and interleukin-2. J Gastroenterol 1998; 33: 39–47.
Pender SL, Fell JM, Chamow SM, Ashkenazi A, MacDonald TT . A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol 1998; 160: 4098–103.
Wen YC, Lee WJ, Tan P, Yang SF, Hsiao M, Lee LM, et al. By inhibiting snail signaling and miR-23a-3p, osthole suppresses the EMT-mediated metastatic ability in prostate cancer. Oncotarget 2015; 6: 21120–36.
Wu SJ . Osthole attenuates inflammatory responses and regulates the expression of inflammatory mediators in HepG2 cells grown in differentiated medium from 3T3-L1 preadipocytes. J Med Food 2015; 18: 972–9.
Yu HP, Liu FC, Tsai YF, Hwang TL . Osthole attenuates hepatic injury in a rodent model of trauma-hemorrhage. PLoS One 2013; 8: e65916.
Liao PC, Chien SC, Ho CL, Wang EI, Lee SC, Kuo YH, et al. Osthole regulates inflammatory mediator expression through modulating NF-kappaB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J Agric Food Chem 2010; 58: 10445–51.
Fusi F, Sgaragli G, Ha le M, Cuong NM, Saponara S . Mechanism of osthole inhibition of vascular Cav1.2 current. Eur J Pharmacol 2012; 680: 22–7.
Ko FN, Wu TS, Liou MJ, Huang TF, Teng CM . Vasorelaxation of rat thoracic aorta caused by osthole isolated from Angelica pubescens. Eur J Pharmacol 1992; 219: 29–34.
Guh JH, Yu SM, Ko FN, Wu TS, Teng CM . Antiproliferative effect in rat vascular smooth muscle cells by osthole, isolated from Angelica pubescens. Eur J Pharmacol 1996; 298: 191–7.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No 81270944 and 81070678), and the Qinglan Project (2014) awarded to Hao LI. Dr Hao LI is an Associate Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information is available on the website of Acta Pharmacologica Sinica.
Supplementary information
Rights and permissions
About this article
Cite this article
Sun, W., Cai, Y., Zhang, Xx. et al. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacol Sin 38, 1120–1128 (2017). https://doi.org/10.1038/aps.2017.71
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.71
Keywords
This article is cited by
-
Osthole prevents tamoxifen-induced liver injury in mice
Acta Pharmacologica Sinica (2019)