Abstract
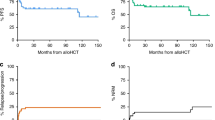
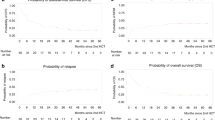
Allogeneic stem cell transplantation (alloSCT) remains the only curative option for CLL patients. Whereas active disease at the time of alloSCT predicts poor outcome, no standard remission-induction regimen exists. We prospectively assessed outcome after cisplatin-containing immune-chemotherapy (R-DHAP) followed by alloSCT in 46 patients (median age 58 years) fulfilling modified European Society for Blood and Marrow Transplantation (EBMT) CLL Transplant Consensus criteria being refractory to or relapsed (R/R) <1 year after fludarabine or <2 years after fludarabine-based immunochemotherapy or R/R with del(17p). Twenty-nine patients received ⩾3 cycles of R-DHAP and sixteen <3 cycles (4 because of disease progression, 8 for toxicity and 4 toxic deaths). Overall rate of response to R-DHAP was 58%, 31 (67%) proceeded to alloSCT after conditioning with fludarabine and 2 Gy TBI. Twenty (65%) remained free from progression at 2 years after alloSCT, including 17 without minimal residual disease. Intention-to-treat 2-year PFS and overall survival of the 46 patients were 42 and 51% (35.5 months median follow-up); del(17p) or fludarabine refractoriness had no impact. R-DHAP followed by alloSCT is a reasonable treatment to be considered for high-risk CLL patients without access or resistance to targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol 2008; 26: 4912–4920.
Dreger P, Schnaiter A, Zenz T, Bottcher S, Rossi M, Paschka P et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood 2013; 121: 3284–3288.
Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia 2013; 27: 362–369.
Khouri IF, Bassett R, Poindexter N, O'Brien S, Bueso-Ramos CE, Hsu Y et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer 2011; 117: 4679–4688.
Michallet M, Socie G, Mohty M, Sobh M, Bay JO, Morisset S et al. Rituximab, fludarabine, and total body irradiation as conditioning regimen before allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukemia: long-term prospective multicenter study. Exp Hematol 2013; 41: 127–133.
Richardson SE, Khan I, Rawstron A, Sudak J, Edwards N, Verfuerth S et al. Risk-stratified adoptive cellular therapy following allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukaemia. Br J Haematol 2013; 160: 640–648.
Scime RTS, Santoro A, Cavallaro AM, Majolino I . Delayed complete hematologic and molecular response in refractory BCLL after allogeneic PBSC transplantation. Blood 1999; 94: 381B.
Schetelig J, Thiede C, Bornhauser M, Schwerdtfeger R, Kiehl M, Beyer J et al. Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: the Cooperative German Transplant Study Group. J Clin Oncol 2003; 21: 2747–2753.
Moreno C, Villamor N, Colomer D, Esteve J, Gine E, Muntanola A et al. Clinical significance of minimal residual disease, as assessed by different techniques, after stem cell transplantation for chronic lymphocytic leukemia. Blood 2006; 107: 4563–4569.
Rondon G, Giralt S, Huh Y, Khouri I, Andersson B, Andreeff M et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant 1996; 18: 669–672.
Mehta J, Powles R, Kulkarni S, Treleaven J, Singhal S . Induction of graft-versus-host disease as immunotherapy of leukemia relapsing after allogeneic transplantation: single-center experience of 32 adult patients. Bone Marrow Transplant 1997; 20: 129–135.
Doney KC, Chauncey T, Appelbaum FR . Allogeneic related donor hematopoietic stem cell transplantation for treatment of chronic lymphocytic leukemia. Bone Marrow Transplant 2002; 29: 817–823.
Farina L, Carniti C, Dodero A, Vendramin A, Raganato A, Spina F et al. Qualitative and quantitative polymerase chain reaction monitoring of minimal residual disease in relapsed chronic lymphocytic leukemia: early assessment can predict long-term outcome after reduced intensity allogeneic transplantation. Haematologica 2009; 94: 654–662.
Bottcher S, Ritgen M, Dreger P . Allogeneic stem cell transplantation for chronic lymphocytic leukemia: lessons to be learned from minimal residual disease studies. Blood Rev 2011; 25: 91–96.
Khouri IF, Lee MS, Saliba RM, Andersson B, Anderlini P, Couriel D et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol 2004; 32: 28–35.
Dreger P, Brand R, Hansz J, Milligan D, Corradini P, Finke J et al. Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia 2003; 17: 841–848.
Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia 2007; 21: 12–17.
Dreger P, Brand R, Milligan D, Corradini P, Finke J, Lambertenghi Deliliers G et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia 2005; 19: 1029–1033.
Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010; 116: 2438–2447.
Majolino I, Ladetto M, Locasciulli A, Drandi D, Benedetti F, Gallamini A et al. High-risk fludarabine-pretreated B-cell chronic lymphocytic leukemia's high response rate following sequential DHAP and alemtuzumab administration though in absence of molecular remission. Med Oncol 2006; 23: 359–368.
Tonino SH, van Gelder M, Eldering E, van Oers MH, Kater AP . R-DHAP is effective in fludarabine-refractory chronic lymphocytic leukemia. Leukemia 2010; 24: 652–654.
Tonino SH, van Laar J, van Oers MH, Wang JY, Eldering E, Kater AP . ROS-mediated upregulation of Noxa overcomes chemoresistance in chronic lymphocytic leukemia. Oncogene 2011; 30: 701–713.
Tonino SH, Mulkens CE, van Laar J, Derks IA, Suo G, Croon-de Boer F et al. Induction of TAp73 by platinum-based compounds to overcome drug resistance in p53 dysfunctional chronic lymphocytic leukemia. Leuk Lymphoma 2015; 56: 2439–2447.
Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4070–4078.
Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1756–1765.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines; new version, updated and corrected, as of December 8, 2008. Blood 2008; 112: 5259.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Vellenga E, van Putten WL, van't Veer MB, Zijlstra JM, Fibbe WE, van Oers MH et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood 2008; 111: 537–543.
Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007; 21: 956–964.
Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 2004; 103: 3278–3281.
Pettitt AR, Matutes E, Oscier D . Alemtuzumab in combination with high-dose methylprednisolone is a logical, feasible and highly active therapeutic regimen in chronic lymphocytic leukaemia patients with p53 defects. Leukemia 2006; 20: 1441–1445.
Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ . Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008; 22: 2048–2053.
Stilgenbauer S, Zenz T . Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2010; 2010: 481–488.
Tam CS, O'Brien S, Plunkett W, Wierda W, Ferrajoli A, Wang X et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab). Blood 2014; 124: 3059–3064.
Tsimberidou AM, Wierda WG, Wen S, Plunkett W, O'Brien S, Kipps TJ et al. Phase I-II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab therapy in aggressive relapsed/refractory chronic lymphocytic leukemia or Richter syndrome. Clin Lymphoma Myeloma Leuk 2013; 13: 568–574.
Durot E, Michallet AS, Lepretre S, Le QH, Leblond V, Delmer A . Platinum and high-dose cytarabine-based regimens are efficient in ultra high/high-risk chronic lymphocytic leukemia and Richter's syndrome: results of a French retrospective multicenter study. Eur J Haematol 2015; 95: 160–167.
Herth I, Dietrich S, Benner A, Hegenbart U, Rieger M, Stadtherr P et al. The impact of allogeneic stem cell transplantation on the natural course of poor-risk chronic lymphocytic leukemia as defined by the EBMT consensus criteria: a retrospective donor versus no donor comparison. Ann Oncol 2014; 25: 200–206.
Michallet M, Sobh M, Milligan D, Morisset S, Niederwieser D, Koza V et al. The impact of HLA matching on long-term transplant outcome after allogeneic hematopoietic stem cell transplantation for CLL: a retrospective study from the EBMT registry. Leukemia 2010; 24: 1725–1731.
Van Gelder M, de Wreede L, Henseler A, van Biezen A, Kröger N, Schetelig J et al. Long-term follow-up data support the curative potential of allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia: a retrospective analysis from the chronic malignancies working party of the EBMT. ASH 2014 annual meeting abstract. Blood 2014; 124: 2561.
Schetelig J, de Wreede L, Moreno C, Smedegaard Andersen N, van Gelder M, Kröger N et al Risk factors for adverse outcome in patients with Chronic Lymphocytic Leukemia (CLL) undergoing Allogeneic Hematopoietic Cell Transplantation (alloHCT): A Retrospective EBMT Analysis. EBMT 2015, annual meeting abstract WP024.
Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J et al. Managing high-risk chronic lymphocytic leukemia during transition to a new treatment era: stem cell transplantation or novel agents? Blood 2014; 124: 3841–3849.
Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371: 213–223.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370: 997–1007.
Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N et al. Outcomes of patients with chronic lymphocytic leukemia (CLL) after discontinuing ibrutinib. Blood 2015; 125: 2062–2067.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015; 125: 2497–2506.
Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2006; 177: 6598–6602.
Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1 selective pressure in T-lymphocytes. Blood 2013; 122: 2539–2549.
Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370: 2286–2294.
Acknowledgements
The data management was financially supported by the Dutch Cancer Foundation (Koningin Wilhelmina Fonds, Amsterdam, The Netherlands). Monique Steijaert, Fokje Spoelstra and Ine Meulendijks (trial and data managers, HOVON Data Center) are acknowledged for their excellent help. Off-label rituximab was generously supplied by Roche Nederland (Woerden, The Netherlands).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
van Gelder, M., van Oers, M., Alemayehu, W. et al. Efficacy of cisplatin-based immunochemotherapy plus alloSCT in high-risk chronic lymphocytic leukemia: final results of a prospective multicenter phase 2 HOVON study. Bone Marrow Transplant 51, 799–806 (2016). https://doi.org/10.1038/bmt.2016.9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/bmt.2016.9
This article is cited by
-
Outcomes of haploidentical stem cell transplantation for chronic lymphocytic leukemia: a retrospective study on behalf of the chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2018)
-
Risk factors for treatment failure after allogeneic transplantation of patients with CLL: a report from the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2017)
-
Transplantation in CLL: what we can learn from a dinosaur
Bone Marrow Transplantation (2016)
-
The addition of ofatumumab to the conditioning regimen does not improve the outcome of patients with high-risk CLL undergoing reduced intensity allogeneic haematopoietic cell transplantation: a pilot trial from the GETH and GELLC (CLL4 trial)
Bone Marrow Transplantation (2016)