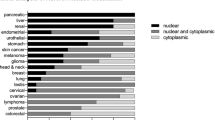
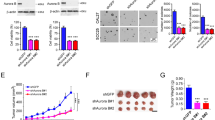
Abstract
Small-molecule inhibitors of the Aurora A and B kinases interfere with mitotic centrosome function and disrupt the mitotic spindle assembly checkpoint resulting in polyploidization and apoptosis of proliferating cells. As such, several Aurora kinase inhibitors are at various stages of clinical development as anticancer agents. To identify candidate apoptosis-sensitizing genes that could be exploited in combination with Aurora kinase inhibitors in malignant glioma, we have carried out global gene expression analysis in a D54MG glioma cell derivative treated with three Aurora kinase inhibitors chosen for their distinctive selectivities: MLN8054 (Aurora A-selective), AZD1152 (Aurora B-selective), and VX-680 (Aurora A/B). The modulation of apoptotic gene expression by p53 under these conditions was ascertained, as p53 expression can be toggled on and off in this D54MG derivative by virtue of a stable, inducible, p53-targeting short hairpin RNA (D54MGshp53). This analysis identified the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor, TRAIL receptor 2 (TRAIL-R2), as an apoptosis-sensitizing gene induced selectively following inhibition of Aurora B. In glioma cell lines where TRAIL-R2 was induced following polyploidization, the sensitivity, kinetics, and magnitude of TRAIL-mediated apoptosis were enhanced. Our data shed light on the apoptotic program induced during polyploidization and suggest that TRAIL-R2 activation is a putative point of therapeutic intervention in combination with inhibitors of Aurora B.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- FACS:
-
fluorescence-activated cell sorting
- FADD:
-
Fas-associated death domain
- IPA:
-
Ingenuity Pathway Analysis
- PE:
-
phycoerythrin
- shRNA:
-
short hairpin RNA
- TRAIL:
-
tumor necrosis factor-related apoptosis-inducing ligand
- TRAIL-R1:
-
tumor necrosis factor-related apoptosis-inducing ligand receptor 1
- TRAIL-R2:
-
tumor necrosis factor-related apoptosis-inducing ligand receptor 2
References
Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005; 65: 2899–2905.
Hu HM, Chuang CK, Lee MJ, Tseng TC, Tang TK . Genomic organization, expression, and chromosome localization of a third aurora-related kinase gene, Aie1. DNA Cell Biol 2000; 19: 679–688.
Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA 2007; 104: 4106–4111.
Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N et al. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol 2007; 27: 4513–4525.
Mortlock AA, Foote KM, Heron NM, Jung FH, Pasquet G, Lohmann JJ et al. Discovery, synthesis, and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J Med Chem 2007; 50: 2213–2224.
Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood 2007; 110: 2034–2040.
Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med 2004; 10: 262–267.
Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C et al. AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res 2007; 13: 3682–3688.
Minn AJ, Boise LH, Thompson CB . Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev 1996; 10: 2621–2631.
Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL et al. A p53-dependent mouse spindle checkpoint. Science 1995; 267: 1353–1356.
Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI . The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res 2006; 66: 7668–7677.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155–162.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–163.
Griffith TS, Rauch CT, Smolak PJ, Waugh JY, Boiani N, Lynch DH et al. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol 1999; 162: 2597–2605.
Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol 2001; 166: 4891–4898.
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 2001; 7: 954–960.
Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer 2005; 92: 1430–1441.
Pollack IF, Erff M, Ashkenazi A . Direct stimulation of apoptotic signaling by soluble Apo2 l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res 2001; 7: 1362–1369.
Roth W, Isenmann S, Naumann U, Kügler S, Bähr M, Dichgans J et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun 1999; 256: 479–483.
Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ . Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res 2000; 60: 847–853.
Warner SL, Munoz RM, Stafford P, Koller E, Hurley LH, Von Hoff DD et al. Comparing Aurora A and Aurora B as molecular targets for growth inhibition of pancreatic cancer cells. Mol Cancer Ther 2006; 5: 2450–2458.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA 2000; 97: 1754–1759.
LeBlanc H, Lawrence DA, Varfolomeev E, Totpal K, Morlan J, Schow P et al. Tumor-cell resistance to death receptor – induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 2002; 8: 274–281.
Wen J, Ramadevi N, Nguyen D, Perkins C, Worthington E, Bhalla K . Antileukemic drugs increase death receptor 5 levels and enhance Apo-2L-induced apoptosis of human acute leukemia cells. Blood 2000; 96: 3900–3906.
Nimmanapalli R, Perkins CL, Orlando M, O'Bryan E, Nguyen D, Bhalla KN . Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res 2001; 61: 759–763.
Buchsbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Zhang S et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res 2003; 9: 3731–3741.
Ohtsuka T, Buchsbaum DJ, Oliver PG, Makhija S, Kimberly RP, Zhou T . Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene 2003; 22: 2034–2044.
Meng RD, El-Deiry WS . p53-independent upregulation of KILLER/DR5 TRAIL receptor expression by glucocorticoids and interferon-gamma. Exp Cell Res 2001; 262: 154–169.
Shankar S, Chen X, Srivastava RK . Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate 2005; 62: 165–186.
Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS et al. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res 1998; 58: 1593–1598.
Röhn TA, Wagenknecht B, Roth W, Naumann U, Gulbins E, Krammer PH et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene 2001; 20: 4128–4137.
Ravi R, Jain AJ, Schulick RD, Pham V, Prouser TS, Allen H et al. Elimination of hepatic metastases of colon cancer cells via p53-independent cross-talk between irinotecan and Apo2 ligand/TRAIL. Cancer Res 2004; 64: 9105–9114.
Khosla S . Minireview: the OPG/RANKL/RANK system. Endocrinology 2001; 142: 5050–5055.
Holen I, Shipman CM . Role of osteoprotegerin (OPG) in cancer. Clin Sci (Lond) 2006; 110: 279–291.
Kuijlen JM, Mooij JJ, Platteel I, Hoving EW, van der Graaf WT, Span MM et al. TRAIL-receptor expression is an independent prognostic factor for survival in patients with a primary glioblastoma multiforme. J Neurooncol 2006; 78: 161–171.
Lin X, Yang J, Chen J, Gunasekera A, Fesik SW, Shen Y . Development of a tightly regulated U6 promoter for shRNA expression. FEBS Lett 2004; 19: 376–380.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57: 289–300.
Berenbaum MC . Criteria for analyzing interactions between biologically active agents. Adv Cancer Res 1981; 35: 269–335.
Acknowledgements
We thank Zhiqin Ji for synthesizing MLN8054 and AZD1152 and Douglas H Steinman for the synthesis of VX-680. We also thank Zehan Chen for technical assistance with the flow cytometric studies carried out here and Joel D Leverson, Chris Tse, and Steven K Davidsen for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by A Ashkenazi
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Li, J., Anderson, M., Tucker, L. et al. Inhibition of Aurora B kinase sensitizes a subset of human glioma cells to TRAIL concomitant with induction of TRAIL-R2. Cell Death Differ 16, 498–511 (2009). https://doi.org/10.1038/cdd.2008.174
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.174
Keywords
This article is cited by
-
RecQL4-Aurora B kinase axis is essential for cellular proliferation, cell cycle progression, and mitotic integrity
Oncogenesis (2018)
-
Aurora kinase inhibitor AZD1152 has an additional effect of platinum on a sequential application at the human ovarian cancer cell line SKOV3
Archives of Gynecology and Obstetrics (2013)
-
Aurora kinase B/C inhibition impairs malignant glioma growth in vivo
Journal of Neuro-Oncology (2012)
-
Type I IFNs signaling and apoptosis resistance in glioblastoma cells
Apoptosis (2011)