Abstract
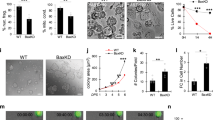
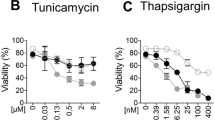
The RNA alphavirus Semliki Forest (SFV) triggers apoptosis in various mammalian cells, but it has remained controversial at what infection stage and by which signalling pathways host cells are killed. Both RNA synthesis-dependent and -independent initiation processes and mitochondrial as well as death receptor signalling pathways have been implicated. Here, we show that SFV-induced apoptosis is initiated at the level of RNA replication or thereafter. Moreover, by expressing antiapoptotic genes from recombinant SFV (replicons) and by using neutralizing reagents and gene-knockout cells, we provide clear evidence that SFV does not require CD95L-, TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)- or tumor necrosis factor-mediated signalling but mitochondrial Bak to trigger cytochrome c release, the fall in the mitochondrial membrane potential, apoptotic protease-activating factor-1/caspase-9 apoptosome formation and caspase-3/-7 activation. Of seven BH3-only proteins tested, only Bid contributed to effective SFV-induced apoptosis. However, caspase-8 activation and Bid cleavage occurred downstream of Bax/Bak, indicating that truncated Bid formation serves to amplify rather than trigger SFV-induced apoptosis. Our data show that SFV sequentially activates a mitochondrial, Bak-mediated, caspase-8-dependent and Bid-mediated death signalling pathway that can be accurately dissected with gene-knockout cells and SFV replicons carrying antiapoptotic genes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- rSFV:
-
recombinant Semliki Forest virus
- SIN:
-
Sindbis virus
- TRAIL:
-
tumor necrosis factor-related apoptosis-inducing ligand
- TNF:
-
tumor necrosis factor
- nsP:
-
non-structural protein
- DISC:
-
death-inducing signalling complex
- FLIP:
-
Fas-associating protein with death domain-like interleukin-1 β-converting enzyme inhibitory protein
- MEF:
-
mouse embryonic fibroblast
- h.p.i.:
-
hours post-infection
- UV:
-
ultraviolet
- SV40:
-
simian virus 40
- DKO:
-
double knockout
- PI:
-
propidium iodide
- Q-VD-OPH:
-
Q-Val-Asp O-Ph, non-O-methylated
- NT:
-
N terminus
- ΔΨm:
-
mitochondrial membrane potential
- MTT:
-
methyl thiazolyl tetrazolium
- Apaf-1:
-
apoptotic protease-activating factor-1
- GFP:
-
green fluorescent protein
- FACS:
-
fluorescence-activated cell sorter
- XIAP:
-
X-linked inhibitor of apoptosis protein
- m.o.i.:
-
multiplicity of infection
- dsRNA:
-
double-stranded RNA
- BH3:
-
Bcl-2 homology domain 3
- PKR:
-
double-stranded RNA-activated protein kinase
- DTT:
-
dithiothreitol
- DMEM:
-
Dulbecco's modified Eagle's medium
- LAMP-1:
-
lysosome associated membrane protein-1
- PMSF:
-
phenylmethylsulfonyl fluoride
- crmA:
-
cytokine response modifier A
- wt:
-
wild type
- DEVD-AMC:
-
acetyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-7-amino-4-methylcoumarin
- DN:
-
dominant negative
- FCS:
-
fetal calf serum
- TMRE:
-
tetramethylrhodamine, ethyl ester, perchlorate
References
Strauss JH, Strauss EG . The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 1984; 58: 491–562.
Atkins GJ, Balluz IM, Glasgow GM, Mabruk MJ, Natale VA, Smyth JM et al. Analysis of the molecular basis of neuropathogenesis of RNA viruses in experimental animals: relevance for human disease? Neuropathol Appl Neurobiol 1994; 20: 91–102.
Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM . Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 1993; 361: 739–742.
Ubol S, Tucker PC, Griffin DE, Hardwick JM . Neurovirulent strains of Alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 protein. Proc Natl Acad Sci USA 1994; 91: 5202–5206.
Rheme C, Ehrengruber MU, Grandgirard D . Alphaviral cytotoxicity and its implication in vector development. Exp Physiol 2004; 90: 45–52.
Levine B, Goldman JE, Jiang HH, Griffin DE, Hardwick JM . Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA 1996; 93: 4810–4815.
Tseng JC, Levin B, Hurtado A, Yee H, Perez de Castro I, Jimenez M et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotech 2004; 22: 70–76.
Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV . Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science 1989; 243: 1188–1191.
Thorburn A . Death receptor-induced cell killing. Cell Signal 2004; 16: 139–144.
Simon NW, Adams JM . Life in balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 2005; 17: 617–625.
Willis SN, Chen L, Dewson G, Wie A, Naik E, Fletcher JI et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 2005; 9: 1294–1305.
Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M et al. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood 2006; 108: 1461–1468.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535.
Green DR, Kroemer G . The pathophysiology of mitochondrial cell death. Science 2004; 305: 626–629.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G . Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta 2004; 1659: 178–189.
Taylor JM, Barry M . Near death experiences: poxvirus regulation of apoptotic death. Virology 2006; 344: 139–150.
Bitzer M, Prinz F, Bauer M, Spiegel M, Neubert WJ, Gregor M et al. Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32). J Virol 1999; 73: 702–708.
Scallan MF, Allsoopp TE, Fazakerley JK . Bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol 1997; 71: 1583–1590.
Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G et al. Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J Virol 1998; 72: 8586–8596.
Moriishi K, Koura M, Matsuura Y . Induction of Bad-mediated apoptosis by Sindbis virus infection: involvement of pro-survival members of the Bcl-2 family. Virology 2001; 292: 258–271.
Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C et al. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J 1998; 17: 1268–1278.
Sarid E, Ben-Moshe T, Kazimirsky G, Weisberg S, Appel E, Kobiler D et al. vFLIP protects PC12 cells from apoptosis induced by Sindbis virus: implications for the role of TNFα. Cell Death Differ 2001; 8: 1224–1231.
Nava V, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM . Sindbis virus induces apoptosis through a caspase-dependent, crmA-sensitive pathway. J Virol 1998; 72: 452–459.
Jan JT, Griffin DE . Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol 1999; 73: 10296–10302.
Zhang P, Samuel CE . Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J Virol 2007; 81: 8192–8200.
Fazakerley JK, Boyd A, Mikkola ML, Kääriäinen L . A single amino acid change in the nuclear localization sequence of the nsP2 protein affects the neurovirulence of Semliki Forest virus. J Virol 2002; 76: 392–396.
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399.
Cowling V, Downward J . Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ 2002; 9: 1046–1056.
Kuwana T, Smith JJ, Muzio M, Dixit VM, Newmeyer DD, Kornbluth S . Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem 1998; 273: 16589–16594.
Desagher S, Martinou JC . Mitochondria as the central control point of apoptosis. Trends Cell Biol 2000; 10: 369–377.
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ . VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 2003; 301: 513–517.
Pardo J, Urban C, Galvez EM, Müller U, Kwon-Chung J, Lobigs M et al. Bak is key in gliotoxin-induced apoptosis and a critical host factor of A. fumigatus virulence in mice. J Cell Biol 2006; 174: 509–519.
Lindenboim L, Kringel S, Braun T, Borner C, Stein R . Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ 2005; 12: 713–723.
Lewis J, Oyler GA, Ueno K, Fannjiang YR, Chau BM, Vornov J et al. Inhibition of virus-induced neuronal apoptosis by Bax. Nat Med 1999; 5: 832–835.
Glasgow GM, Killen HM, Liljeström P, Sheahan BJ, Atkins GJ . A single amino acid change in the E2 spike protein of a virulent strain of Semliki Forest virus attenuates pathogenicity. J Gen Virol 1994; 75: 663–668.
Garmashova N, Gorchakov R, Frolova E, Frolov I . Sindbis virus non-structural protein nsP2 is cytotoxic and inhibits cellular transcription. J Virol 2006; 80: 5686–5696.
Adams KW, Cooper GM . Rapid turnover of MCL-1 couples translation to cell survival and apoptosis. J Biol Chem 2007; 282: 6192–6200.
Meylan E, Tschopp J, Karin M . Intracellular pattern recognition receptors in the host response. Nature 2006; 442: 39–44.
Egger L, Schneider J, Rhême C, Tapernoux M, Häcki J, Borner C . Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase inhibiting conditions. Cell Death Differ 2003; 10: 1188–1203.
Acknowledgements
We thank A Strasser for the Apaf-1−/−, Bim−/−, Bad−/−, Bik−/−, Bim/Bad and Bim/Bid DKO MEFs; A Villunger for the Bmf−/−, Puma−/− and Noxa−/− MEFs; the late SJ Korsmeyer for the Bid−/−, Bax−/−, Bak−/− and Bax/Bak DKO MEFs; YA Lazebnik for the caspase-9−/− MEFs and the caspase-9 DN (C287S) construct; DCS Huang for the anti-Bid antibody and the FLAG-crmA construct; H Walczak and M Sprick for the leucine zipper-TRAIL, anti-TRAIL-R1 and TRAIL-R2-Fc; J Tschopp for the caspase-8 DN (C360S) construct; J Silke for the XIAP-FLAG construct; R Flavell for caspase-3−/− and caspase-3/-7 DKO cells; J Pavlovic for the anti-SFV-C antibody. MU Ehrengruber for the BHK-21 cells and the pSFV2subGFP construct and J-C Martinou for the recombinant tBid.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Piacentini
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Urban, C., Rhême, C., Maerz, S. et al. Apoptosis induced by Semliki Forest virus is RNA replication dependent and mediated via Bak. Cell Death Differ 15, 1396–1407 (2008). https://doi.org/10.1038/cdd.2008.61
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.61