Abstract
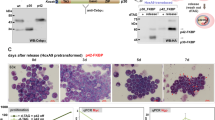
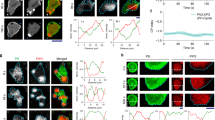
Mouse embryonic stem (ES) cells remain pluripotent in vitro when grown in the presence of leukemia inhibitory factor (LIF) cytokine. LIF starvation leads to cell commitment, and part of the ES-derived differentiated cells die by apoptosis together with caspase3-cleavage and p38α activation. Inhibition of p38 activity by chemical compounds (PD169316 and SB203580), along with LIF withdrawal, leads to different outcomes on cell apoptosis, giving the opportunity to study the influence of apoptosis on cell differentiation. By gene profiling studies on ES-derived differentiated cells treated or not with these inhibitors, we have characterized the common and specific set of genes modulated by each inhibitor. We have also identified key genes that might account for their different survival effects. In addition, we have demonstrated that some genes, similarly regulated by both inhibitors (upregulated as Bcl2, Id2, Cd24a or downregulated as Nodal), are bona fide p38α targets involved in neurogenesis and found a correlation with their expression profiles and the onset of neuronal differentiation triggered upon retinoic acid treatment. We also showed, in an embryoid body differentiation protocol, that overexpression of EGFP (enhanced green fluorescent protein)–BCL2 fusion protein and repression of p38α are essential to increase formation of TUJ1-positive neuronal cell networks along with an increase in Map2-expressing cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- EBs:
-
embryoid bodies
- ES:
-
embryonic stem
- ERK2:
-
extracellular-signal regulated kinase 2
- EGFP:
-
enhanced green fluorescent protein
- LIF:
-
leukemia inhibitory factor
- MAP2:
-
microtubule-associated protein 2
- MAPK:
-
mitogen-activated protein kinase
- RA:
-
retinoic acid
- RTQ-PCR:
-
real-time quantitative-polymerase chain reaction
References
Shellard J, Perreau J, Brulet P . Role of leukemia inhibitory factor during mammalian development. Eur Cytokine Netw 1996; 7: 699–712.
Burdon T, Smith A, Savatier P . Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol 2002; 12: 432.
Metcalf D . The unsolved enigmas of leukemia inhibitory factor. Stem Cells 2003; 21: 5–14.
Babinet C, Cohen-Tannoudji M . Genome engineering via homologous recombination in mouse embryonic stem (ES) cells: an amazingly versatile tool for the study of mammalian biology. An Acad Bras Cienc 2001; 73: 365–383.
Smith AG . Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 2001; 17: 435–462.
Wobus AM, Boheler KR . Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev 2005; 85: 635–678.
Duval D, Reinhardt B, Kedinger C, Boeuf H . Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF) -dependent embryonic stem cell survival. FASEB J 2000; 14: 1577–1584.
Duval D, Malaise M, Reinhardt B, Kedinger C, Boeuf HA . p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ 2004; 11: 331–341.
Duval D, Trouillas M, Thibault C, Dembele D, Diemunsch F, Reinhardt B et al. Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ 2006; 13: 564–575.
Li M, Pevny L, Lovell-Badge R, Smith A . Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol 1998; 8: 971–974.
Ying QL, Smith AG . Defined conditions for neural commitment and differentiation. Methods Enzymol 2003; 365: 327–341.
Bouhon IA, Kato H, Chandran S, Allen ND . Neural differentiation of mouse embryonic stem cells in chemically defined medium. Brain Res Bull 2005; 68: 62–75.
Gossrau G, Thiele J, Konang R, Schmandt T, Brustle O . Bone morphogenetic protein-mediated modulation of lineage diversification during neural differentiation of embryonic stem cells. Stem Cells 2007; 25: 939–949.
Aouadi M, Bost F, Caron L, Laurent K, Le Marchand Brustel Y, Binetruy B . p38 mitogen-activated protein kinase activity commits embryonic stem cells to either neurogenesis or cardiomyogenesis. Stem Cells 2006; 24: 1399–1406.
Keren A, Tamir Y, Bengal E . The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol 2006; 252: 224–230.
Binetruy B, Heasley L, Bost F, Caron L, Aouadi M . Concise review: regulation of embryonic stem cell lineage commitment by mitogen-activated protein kinases. Stem Cells 2007; 25: 1090–1095.
Kumar S, Boehm J, Lee JC . p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2003; 2: 717–726.
Abraham RT . MAPKAP kinase-2; three's company at the G(2) checkpoint. Mol Cell 2005; 17: 163–164.
Horstmann S, Kahle PJ, Borasio GD . Inhibitors of p38 mitogen-activated protein kinase promote neuronal survival in vitro. J Neurosci Res 1998; 52: 483–490.
Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X et al. p38 kinase is activated in the Alzheimer's disease brain. J Neurochem 1999; 72: 2053–2058.
Culbert AA, Skaper SD, Howlett DR, Evans NA, Facci L, Soden PE et al. MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J Biol Chem 2006; 281: 23658–23667.
Niu W, Huang C, Nawaz Z, Levy M, Somwar R, Li D et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem 2003; 278: 17953–17962.
Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ . The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol 2004; 75: 1173–1182.
Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R . Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 2004; 24: 6539–6549.
Lee ER, McCool KW, Murdoch FE, Fritsch MK . Dynamic changes in histone H3 phosphoacetylation during early embryonic stem cell differentiation are directly mediated by mitogen- and stress-activated protein kinase 1 via activation of MAPK pathways. J Biol Chem 2006; 281: 21162–21172.
Saklatvala J . The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol 2004; 4: 372–377.
Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel CA . Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med 2000; 191: 859–870.
Ihle JN . The challenges of translating knockout phenotypes into gene function. Cell 2000; 102: 131–134.
Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F . Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 2006; 88: 1091–1098.
Ying QL, Stavridis M, Griffiths D, Li M, Smith A . Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol 2003; 21: 183–186.
Chung S, Shin BS, Hedlund E, Pruszak J, Ferree A, Kang UJ et al. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem 2006; 97: 1467–1480.
Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S et al. Differences between human and mouse embryonic stem cells. Dev Biol 2004; 269: 360–380.
Pfendler KC, Catuar CS, Meneses JJ, Pedersen RA . Overexpression of Nodal promotes differentiation of mouse embryonic stem cells into mesoderm and endoderm at the expense of neuroectoderm formation. Stem Cells Dev 2005; 14: 162–172.
Davies SP, Reddy H, Caivano M, Cohen P . Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351: 95–105.
Avitzour M, Diskin R, Raboy B, Askari N, Engelberg D, Livnah O . Intrinsically active variants of all human p38 isoforms. FEBS J 2007; 274: 963–975.
Palmqvist L, Glover CH, Hsu L, Lu M, Bossen B, Piret JM et al. Correlation of murine embryonic stem cell gene expression profiles with functional measures of pluripotency. Stem Cells 2005; 23: 663–680.
Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C et al. Dissecting self-renewal in stem cells with RNA interference. Nature 2006; 442: 533–538.
Jones FS, Meech R . Knockout of REST/NRSF shows that the protein is a potent repressor of neuronally expressed genes in non-neural tissues. Bioessays 1999; 21: 372–376.
Greenway DJ, Street M, Jeffries A, Buckley NJ . RE1 silencing transcription factor maintains a repressive chromatin environment in embryonic hippocampal neural stem cells. Stem Cells 2007; 25: 354–363.
Lu CC, Robertson EJ . Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol 2004; 273: 149–159.
Ying QL, Nichols J, Chambers I, Smith A . BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003; 115: 281–292.
Yamane T, Dylla SJ, Muijtjens M, Weissman IL . Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc Natl Acad Sci USA 2005; 102: 3312–3317.
Peddada S, Yasui DH, LaSalle JM . Inhibitors of differentiation (ID1, ID2, ID3 and ID4) genes are neuronal targets of MeCP2 that are elevated in Rett syndrome. Hum Mol Genet 2006; 15: 2003–2014.
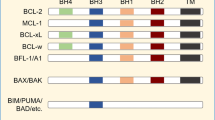
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–656.
Zhang KZ, Westberg JA, Holtta E, Andersson LC . BCL2 regulates neural differentiation. Proc Natl Acad Sci USA 1996; 93: 4504–4508.
Suzuki A, Tsutomi Y . Bcl-2 accelerates the neuronal differentiation: new evidence approaching to the biofunction of bcl-2 in the neuronal system. Brain Res 1998; 801: 59–66.
Sato N, Sakuma C, Kato H, Milligan CE, Oppenheim RW, Yaginuma H . Bcl-2 rescues motoneurons from early cell death in the cervical spinal cord of the chicken embryo. J Neurobiol 2002; 53: 381–390.
Lowell S, Benchoua A, Heavey B, Smith AG . Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol 2006; 4: e121.
Sumantran VN, Brederlau A, Funa K . BMP-6 and retinoic acid synergistically differentiate the IMR-32 human neuroblastoma cells. Anticancer Res 2003; 23: 1297–1303.
Niizuma H, Nakamura Y, Ozaki T, Nakanishi H, Ohira M, Isogai E et al. Bcl-2 is a key regulator for the retinoic acid-induced apoptotic cell death in neuroblastoma. Oncogene 2006; 25: 5046–5055.
Acknowledgements
We thank all members of the UMR-CNRS-5164-CIRID and, in particular, Jean François Moreau for helpful insights and a careful reading of the paper. We thank C Gabel for the gift of the ES WT and ES p38α−/− cell lines, A Smith for the gift of the ES CGR8 WT and E14TG2aSox1-EGFP ES cell lines, A Dierich for the gift of the S1 ES cell line, R Kemler for the gift of the D3 ES cell line and Dr Miyazaki for the pCXN2 expression vector. We thank V Pitard, M Landry and B Guillotin, respectively, for very good advices concerning the flow cytometry experiments, the characterization of neuronal cells and RTQ-PCR experiments. Thanks also to E Préchais for preliminary data concerning the validation of the p38α target genes. HB thanks P Dubus for constant support and reading of the paper. This work was founded by the European consortium FungenES (6th Framework, project no LSHG-CT-2003-503494, Coordinator: Professor J Hescheler), CNRS, the Ministere of Research (Affymetrix microarray grant, IGBMC genopole platform, Strasbourg), University of Bordeaux 2, the Ligue National contre le Cancer, Comités Aquitaine-Charentes, the Region Aquitaine and the IFR66. MT, OF and MG were financed by a FungenES fellowship and CS by a Region Aquitaine fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by R De Maria
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Trouillas, M., Saucourt, C., Duval, D. et al. Bcl2, a transcriptional target of p38α, is critical for neuronal commitment of mouse embryonic stem cells. Cell Death Differ 15, 1450–1459 (2008). https://doi.org/10.1038/cdd.2008.63
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.63
Keywords
This article is cited by
-
Phospholipase D1 Signaling: Essential Roles in Neural Stem Cell Differentiation
Journal of Molecular Neuroscience (2018)
-
MicroRNA-128-3p Protects Mouse Against Cerebral Ischemia Through Reducing p38α Mitogen-Activated Protein Kinase Activity
Journal of Molecular Neuroscience (2017)
-
Scutellarin Alleviates Behavioral Deficits in a Mouse Model of Multiple Sclerosis, Possibly Through Protecting Neural Stem Cells
Journal of Molecular Neuroscience (2016)
-
Phospholipase D1 Increases Bcl-2 Expression During Neuronal Differentiation of Rat Neural Stem Cells
Molecular Neurobiology (2015)
-
A p38mapk-p53 cascade regulates mesodermal differentiation and neurogenesis of embryonic stem cells
Cell Death & Disease (2013)