Abstract
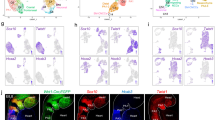
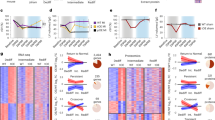
Different cardiac stem/progenitor cells have been recently identified in the post-natal heart. We describe here the identification, clonal expansion and characterization of self-renewing progenitors that differ from those previously described for high spontaneous cardiac differentiation. Unique coexpression of endothelial and pericyte markers identify these cells as cardiac mesoangioblasts and allow prospective isolation and clonal expansion from the juvenile mouse ventricle. Cardiac mesoangioblasts express many cardiac transcription factors and spontaneously differentiate into beating cardiomyocytes that assemble mature sarcomeres and express typical cardiac ion channels. Cells similarly isolated from the atrium do not spontaneously differentiate. When injected into the ventricle after coronary artery ligation, cardiac mesoangioblasts efficiently generate new myocardium in the peripheral area of the necrotic zone, as they do when grafted in the embryonic chick heart. These data identify cardiac mesoangioblasts as committed progenitors, downstream of earlier stem/progenitor cells and suitable for the cell therapy of a subset of juvenile cardiac diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ANF:
-
atrial-natriuretic-peptide
- AP:
-
alkaline phosphatase
- SMA:
-
smooth α actin
- vWF:
-
von Willebrand factor
- CAL:
-
coronary artery ligation
- HH:
-
Hamilton Hamburger
References
Olson E . A decade of discoveries in cardiac biology. Nat Med 2004; 10: 467–474.
Beltrami A, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114: 763–776.
Oh H, Bradfute S, Gallardo T, Nakamura T, Gaussin V, Mishina Y et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 2003; 100: 12313–12318.
Laugwitz K, Moretti A, Lam J, Gruber P, Chen Y, Woodard S et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005; 433: 647–653.
Messina E, Angelis LD, Frati G, Morrone S, Chimenti S, Fiordaliso F et al. Isolation and expansion of adult cardiac stem cell from human and murine heart. Circ Res 2004; 95: 911–921.
Kocher A, Schuster M, Szabolcs M, Takuma S, Burkhoff D, Wang J et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–436.
Galli D, Innocenzi A, Staszewsky L, Zanetta L, Sampaolesi M, Bai A et al. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms. Arterioscler Thromb Vasc Biol 2005; 25: 692–697.
Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006; 444: 574–579.
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L et al. Pericytes of human skeletal muscle are myogenic precursors, distinct from satellite cells, and efficiently repair dystrophic muscle. Nat Cell Biol 2007; 9: 255–267.
Minasi M, Riminucci M, Angelis LD, Borello U, Berarducci B, Innocenzi A et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 2002; 129: 2773–2783.
Nuss HB, Marban E . Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol 1994; 479: 265–279.
Protas L, Barbuti A, Qu J, Rybin VO, Palmiter RD, Steinberg SF et al. Neuropeptide Y is an essential in vivo developmental regulator of cardiac ICa L. Circ Res 2003; 93: 972–979.
Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D et al. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 2004; 559: 103–120.
Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ . Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res 1996; 79: 79–85.
Takemura H, Yasui K, Opthof T, Niwa N, Horiba M, Shimizu A et al. Subtype switching of L-Type Ca2+ channel from Cav1.3 to Cav1.2 in embryonic murine ventricle. Circ J 2005; 69: 1405–1411.
Moretti A, Caron L, Nakano A, Lam J, Bernshausen A, Chen Y et al. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006; 127: 1151–1165.
Wu S, Fujiwara Y, Cibulsky S, Clapham D, Lien C, Schultheiss T et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 2006; 127: 1137–1150.
Badorff C, Brandes R, Popp R, Rupp S, Urbich C, Aicher A et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 2003; 107: 1024–1032.
Nygren J, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004; 10: 494–501.
Zhang S, Wang D, Estrov Z, Raj S, Willerson J, Yeh E . Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation 2004; 110: 3803–3807.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S, Li B et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–705.
Durocher D, Charron F, Warren R, Schwartz R, Nemer M . The cardiac transcription factors Nkx2.5 and GATA-4 are mutual cofactors. EMBO 1997; 16: 5687–5696.
Kitajima S, Takagi A, Inoue T, Saga Y . MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 2000; 127: 3215–3226.
Condorelli G, Borello U, Angelis LD, Latronico M, Sirabella D, Coletta M et al. Cardiomyocytes induce endothelial cells to tras-differentiate into cardiac clones: implications for myocardium regeneration. Proc Natl Acad Sci USA 2001; 98: 10733–10738.
Wang T, Xu Z, Jiang W, Ma A . Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol 2006; 109: 74–81.
Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren J et al. Engrafment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med 2006; 203: 2315–2327.
Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001; 108: 407–414.
Menard C, Hagege A, Agbulut O, Barro M, Morichetti M, Brasselet C et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet 2005; 366: 1005–1012.
Sahn DJ, De Maria A, Kisslo J, Weyman A . Recommendations regarding quantitation in M-mode echocardiography; results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083.
Schiller NB, Shah PM, Crawford M, De Maria A, Devereux R, Feigenbaum H et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography American Society of Echocardiography Committee on Standards, subcommittee on Quantitation of Two Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367.
Acknowledgements
This work was supported by grants from Fondation Leducq, European Community (European Vascular Genomics Network, Heart Repair, Nax and Normacor), Italian Ministries of Health and Research, Firb and CARIPLO. Beatriz G Galvez was supported by a Program 3+3 Fellowship from the Centro Nacional de Investigaciones Cardiovasculares (CNIC), Spain. We thank the microscope service of the san Raffaele foundation for help with the images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by RA Knight
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Galvez, B., Sampaolesi, M., Barbuti, A. et al. Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle. Cell Death Differ 15, 1417–1428 (2008). https://doi.org/10.1038/cdd.2008.75
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.75
Keywords
This article is cited by
-
Adipokines disrupt cardiac differentiation and cardiomyocyte survival
International Journal of Obesity (2020)
-
Bmi1 + cardiac progenitor cells contribute to myocardial repair following acute injury
Stem Cell Research & Therapy (2016)
-
Cardiac Bmi1 + cells contribute to myocardial renewal in the murine adult heart
Stem Cell Research & Therapy (2015)
-
Low-Intensity Pulsed Ultrasound Improves the Functional Properties of Cardiac Mesoangioblasts
Stem Cell Reviews and Reports (2015)
-
Fate choice of post-natal mesoderm progenitors: skeletal versus cardiac muscle plasticity
Cellular and Molecular Life Sciences (2014)