Abstract
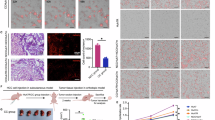
Macroautophagy (called autophagy hereafter) is a catabolic process activated by various types of stress, most notably by nutrient deprivation. The autophagic degradation of intracellular macromolecules provides metabolic support for the cell; however, this physiological process can also initiate a form of cell death (type 2 programmed cell death). Here we report that oxygen deprivation can activate the autophagic pathway in human cancer cell lines. We observed that hypoxia induced distinct cellular changes characteristic of autophagy such as an increase in cytoplasmic acidic vesicles, and processing and cellular localization of microtubule-associated protein-1 light chain 3. Oxygen deprivation-induced autophagy did not require nutrient deprivation, hypoxia-inducible factor-1 (HIF-1) activity, or expression of the HIF-1 target gene BNIP3 (Bcl-2 adenovirus E1a nineteen kilodalton interacting protein 3) or BNIP3L (BNIP3 like protein). Hypoxia-induced autophagy involved the activity of 5′-AMP-activated protein kinase (AMPK). Finally, we determined that cells lacking the autophagy gene ATG5 were unable to activate the autophagic machinery in hypoxia, had decreased oxygen consumption and increased glucose uptake under hypoxia, had increased survival in hypoxic environments, and exhibited accelerated growth as xenografted tumors. Together, these findings suggest that the autophagic degradation of cellular macromolecules contributes to the energetic balance governed by AMPK, and that suppression of autophagy in transformed cells can increase both resistance to hypoxic stress and tumorigenicity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AVO:
-
acidic vesicular organelle
- HIF-1:
-
hypoxia-inducible factor-1
- AMPK:
-
5′-AMP-activated protein kinase
- LC3:
-
microtubule-associated protein-1 light chain 3
- MEF:
-
mouse embryo fibroblast
- mTOR:
-
mammalian target of rapamycin
- TSC:
-
tuberous sclerosis complex
- pVHL:
-
von Hippel-Lindau protein
References
Vaupel P, Mayer A . Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26: 225–239.
Evans SM, Hahn SM, Magarelli DP, Koch CJ . Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol 2001; 24: 467–472.
Hockel M, Schlenger K, Hockel S, Vaupel P . Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res 1999; 59: 4525–4528.
Brunelle JK, Shroff EH, Perlman H, Strasser A, Moraes CT, Flavell RA et al. Loss of Mcl-1 protein and inhibition of electron transport chain together induce anoxic cell death. Mol Cell Biol 2007; 27: 1222–1235.
Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC . Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res 2005; 65: 3171–3178.
Santore MT, McClintock DS, Lee VY, Budinger GR, Chandel NS . Anoxia-induced apoptosis occurs through a mitochondria-dependent pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002; 282: L727–L734.
Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF . BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 2007; 27: 6229–6242.
Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 2000; 20: 5454–5468.
Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S . Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res 2004; 64: 4286–4293.
Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S . Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene 2005; 24: 980–991.
Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 2007; 14: 146–157.
Hardie DG . AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8: 774–785.
Hardie DG . Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179–5183.
Kwiatkowski DJ, Manning BD . Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 2005; 14 (Spec No. 2) R251–R258.
Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 2006; 26: 5336–5347.
Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC . Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 2006; 21: 521–531.
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 2007; 100: 914–922.
Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 2006; 281: 34870–34879.
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G . Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8: 741–752.
Klionsky DJ . Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007; 8: 931–937.
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD et al. A protein conjugation system essential for autophagy. Nature 1998; 395: 395–398.
Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M et al. The energy sensing LKB1–AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007; 9: 218–224.
Yue Z, Jin S, Yang C, Levine AJ, Heintz N . Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 2003; 100: 15077–15082.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–676.
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237–248.
Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006; 10: 51–64.
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 2007; 21: 1621–1635.
Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 2007; 21: 1367–1381.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6: 1221–1228.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304: 1500–1502.
Klionsky DJ, Cuervo AM, Seglen PO . Methods for monitoring autophagy from yeast to human. Autophagy 2007; 3: 181–206.
Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P et al. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol Cell Biol 2001; 21: 3436–3444.
Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008; 4: 195–204.
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 2008; 283: 10892–10903.
Bacon AL, Fox S, Turley H, Harris AL . Selective silencing of the hypoxia-inducible factor 1 target gene BNIP3 by histone deacetylation and methylation in colorectal cancer. Oncogene 2007; 26: 132–141.
Murai M, Toyota M, Suzuki H, Satoh A, Sasaki Y, Akino K et al. Aberrant methylation and silencing of the BNIP3 gene in colorectal and gastric cancer. Clin Cancer Res 2005; 11: 1021–1027.
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC . HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006; 3: 187–197.
Yorimitsu T, Klionsky DJ . Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy 2007; 3: 160–162.
Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 2004; 64: 5943–5947.
Hosokawa N, Hara Y, Mizushima N . Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett 2006; 580: 2623–2629.
Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 2001; 61: 439–444.
George MD, Baba M, Scott SV, Mizushima N, Garrison BS, Ohsumi Y et al. Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways. Mol Biol Cell 2000; 11: 969–982.
Gatenby RA, Gillies RJ . Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891–899.
Laderoute KR, Calaoagan JM, Gustafson-Brown C, Knapp AM, Li GC, Mendonca HL et al. The response of c-jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1 alpha dependent. Mol Cell Biol 2002; 22: 2515–2523.
Acknowledgements
We are grateful to N Mizushima, D Kwiatkowski, and A Koong for cell lines and reagents. This work was funded by the NCI (NCD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by N Chandel
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Papandreou, I., Lim, A., Laderoute, K. et al. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ 15, 1572–1581 (2008). https://doi.org/10.1038/cdd.2008.84
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2008.84
Keywords
This article is cited by
-
REST-dependent downregulation of von Hippel-Lindau tumor suppressor promotes autophagy in SHH-medulloblastoma
Scientific Reports (2024)
-
Hypoxia is fine-tuned by Hif-1α and regulates mesendoderm differentiation through the Wnt/β-Catenin pathway
BMC Biology (2022)
-
Hypoxia-induced HIF1α activation regulates small extracellular vesicle release in human embryonic kidney cells
Scientific Reports (2022)
-
BNIP3L/NIX-mediated mitophagy: molecular mechanisms and implications for human disease
Cell Death & Disease (2021)
-
High-throughput screening for natural compound-based autophagy modulators reveals novel chemotherapeutic mode of action for arzanol
Cell Death & Disease (2021)