Abstract
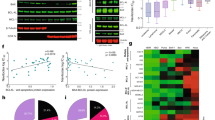
Bcl-2 has been shown to promote survival of cancer cells by maintaining a slight pro-oxidant state through elevated mitochondrial respiration during basal conditions. On oxidative stress, Bcl-2 moderates mitochondrial respiration through cytochrome c oxidase (COX) activity to prevent an excessive buildup of reactive oxygen species (ROS) by-production from electron transport activities. However, the underlying molecular mechanism(s) of Bcl-2-mediated ROS regulation and its impact on carcinogenesis remain unclear. In this study, we show that Bcl-2 expression positively influences the targeting of nuclear-encoded COX Va and Vb to the mitochondria of cancer cells. In addition, evidence is presented in support of a protein–protein interaction between COX Va and Bcl-2, involving the BH2 domain of Bcl-2. Interestingly, episodes of serum withdrawal, glucose deprivation or hypoxia aimed at inducing early oxidative stress triggered Bcl-2-overexpressing cells to preserve mitochondrial levels of COX Va while depressing COX Vb, whereas the reverse was observed in mock-transfected cells. The unique manner in which Bcl-2 adjusted COX subunits during these physiological stress triggers had a profound impact on the resultant decrease in COX activity and maintenance of mitochondrial ROS levels, thus delineating a novel mechanism for the homeostatic role of Bcl-2 in the redox biology and metabolism of cancer cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ATP:
-
adenosine triphosphate
- Bak:
-
Bcl-2 antagonist/killer-1
- Bax:
-
Bcl-2 associated X protein
- Bcl-2:
-
B-cell lymphoma/leukemia-2
- BN-PAGE:
-
blue native polyacrylamide gel electrophoresis
- COX:
-
cytochrome c oxidase
- CuZn SOD:
-
copper/zinc superoxide dismutase
- HIF-1:
-
hypoxia-inducible factor 1
- O2-:
-
superoxide
- RLU:
-
relative light unit
- ROS:
-
reactive oxygen species
- Tom20:
-
translocase of outer membrane 20
References
Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O et al. p53 regulates mitochondrial respiration. Science 2006; 312: 1650–1653.
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL . HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007; 129: 111–122.
Dowhan W, Bibus CR, Schatz G . The cytoplasmically-made subunit IV is necessary for assembly of cytochrome c oxidase in yeast. EMBO J 1985; 4: 179–184.
Trueblood CE, Poyton RO . Differential effectiveness of yeast cytochrome c oxidase subunit genes results from differences in expression not function. Mol Cell Biol 1987; 7: 3520–3526.
Burke PV, Raitt DC, Allen LA, Kellogg EA, Poyton RO . Effects of oxygen concentration on the expression of cytochrome c and cytochrome c oxidase genes in yeast. J Biol Chem 1997; 272: 14705–14712.
Bunn HF, Poyton RO . Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 1996; 76: 839–885.
Dagsgaard C, Taylor LE, O’Brien KM, Poyton RO . Effects of anoxia and the mitochondrion on expression of aerobic nuclear COX genes in yeast: evidence for a signaling pathway from the mitochondrial genome to the nucleus. J Biol Chem 2001; 276: 7593–7601.
Poyton RO, Burke PV . Oxygen regulated transcription of cytochrome c and cytochrome c oxidase genes in yeast. Biochim Biophys Acta 1992; 1101: 252–256.
Williams SL, Valnot I, Rustin P, Taanman JW . Cytochrome c oxidase subassemblies in fibroblast cultures from patients carrying mutations in COX10, SCO1, or SURF1. J Biol Chem 2004; 279: 7462–7469.
Nijtmans LG, Taanman JW, Muijsers AO, Speijer D, Van den Bogert C . Assembly of cytochrome-c oxidase in cultured human cells. Eur J Biochem 1998; 254: 389–394.
Wu H, Rao GN, Dai B, Singh P . Autocrine gastrins in colon cancer cells up-regulate cytochrome c oxidase Vb and down-regulate efflux of cytochrome c and activation of caspase-3. J Biol Chem 2000; 275: 32491–32498.
Wu H, Owlia A, Singh P . Precursor peptide progastrin(1–80) reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP. Am J Physiol Gastrointest Liver Physiol 2003; 285: G1097–G1110.
Fu WN, Shang C, Huang DF, Xu ZM, Sun XH, Sun KL . Average-12.9 chromosome imbalances coupling with 15 differential expression genes possibly involved in the carcinogenesis, progression and metastasis of supraglottic laryngeal squamous cell cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2006; 23: 7–11.
Bini L, Magi B, Marzocchi B, Arcuri F, Tripodi S, Cintorino M et al. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 1997; 18: 2832–2841.
Melis R, White R . Characterization of colonic polyps by two-dimensional gel electrophoresis. Electrophoresis 1999; 20: 1055–1064.
Herrmann PC, Gillespie JW, Charboneau L, Bichsel VE, Paweletz CP, Calvert VS et al. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics 2003; 3: 1801–1810.
Vijayasarathy C, Biunno I, Lenka N, Yang M, Basu A, Hall IP et al. Variations in the subunit content and catalytic activity of the cytochrome c oxidase complex from different tissues and different cardiac compartments. Biochim Biophys Acta 1998; 1371: 71–82.
Campian JL, Gao X, Qian M, Eaton JW . Cytochrome C oxidase activity and oxygen tolerance. J Biol Chem 2007; 282: 12430–12438.
Clement MV, Stamenkovic I . Superoxide anion is a natural inhibitor of FAS-mediated cell death. Embo J 1996; 15: 216–225.
Clement MV, Hirpara JL, Pervaiz S . Decrease in intracellular superoxide sensitizes Bcl-2-overexpressing tumor cells to receptor and drug-induced apoptosis independent of the mitochondria. Cell Death Differ 2003; 10: 1273–1285.
Piantadosi CA, Suliman HB . Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem 2006; 281: 324–333.
Hirai I, Wang HG . Survival-factor-induced phosphorylation of Bad results in its dissociation from Bcl-x(L) but not Bcl-2. Biochem J 2001; 359: 345–352.
Harris MH, Thompson CB . The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 2000; 7: 1182–1191.
Steinman HM . The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem 1995; 270: 3487–3490.
Chen ZX, Pervaiz S . Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ 2007; 14: 1617–1627.
Frezza C, Cipolat S, Scorrano L . Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2007; 2: 287–295.
Su X, Dowhan W . Translational regulation of nuclear gene COX4 expression by mitochondrial content of phosphatidylglycerol and cardiolipin in Saccharomyces cerevisiae. Mol Cell Biol 2006; 26: 743–753.
Brix J, Rudiger S, Bukau B, Schneider-Mergener J, Pfanner N . Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J Biol Chem 1999; 274: 16522–16530.
Motz C, Martin H, Krimmer T, Rassow J . Bcl-2 and porin follow different pathways of TOM-dependent insertion into the mitochondrial outer membrane. J Mol Biol 2002; 323: 729–738.
Yin XM, Oltvai ZN, Korsmeyer SJ . BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 1994; 369: 321–323.
Wright RM, Poyton RO . Release of two Saccharomyces cerevisiae cytochrome genes, COX6 and CYC1, from glucose repression requires the SNF1 and SSN6 gene products. Mol Cell Biol 1990; 10: 1297–1300.
Wright RM, Rosenzweig B, Poyton RO . Organization and expression of the COX6 genetic locus in Saccharomyces cerevisiae: multiple mRNAs with different 3′ termini are transcribed from COX6 and regulated differentially. Nucleic Acids Res 1989; 17: 1103–1120.
Deng X, Gao F, Flagg T, Anderson J, May WS . Bcl2's flexible loop domain regulates p53 binding and survival. Mol Cell Biol 2006; 26: 4421–4434.
Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH et al. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem 2004; 279: 15968–15974.
Krahenbuhl S, Chang M, Brass EP, Hoppel CL . Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem 1991; 266: 20998–21003.
Daley E, Wilkie D, Loesch A, Hargreaves IP, Kendall DA, Pilkington GJ et al. Chlorimipramine: a novel anticancer agent with a mitochondrial target. Biochem Biophys Res Commun 2005; 328: 623–632.
Hirpara JL, Seyed MA, Loh KW, Dong H, Kini RM, Pervaiz S . Induction of mitochondrial permeability transition and cytochrome C release in the absence of caspase activation is insufficient for effective apoptosis in human leukemia cells. Blood 2000; 95: 1773–1780.
Acknowledgements
This work is supported by grants from the Biomedical Research Council of Singapore (R-185–000–164–305), and the NUS Academic Research Fund, Singapore (R-185–000–136–112) to SP. The NPC cell lines were gifts from Lo Kwok-wai (The Chinese University of Hong Kong) and Hsieh Wen-son (John Hopkins Medical Institute, USA). We thank Thomas Loh (National University Hospital, Singapore) and Jayshree Hirpara (National University of Singapore) for providing the lymphoma samples and for excellent technical assistance with the samples, respectively. ZXC designed, performed the experiments and wrote the paper. SP designed the experiments and wrote the paper. Both authors discussed the results and commented on the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by L Scorrano
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, Z., Pervaiz, S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ 17, 408–420 (2010). https://doi.org/10.1038/cdd.2009.132
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2009.132
Keywords
This article is cited by
-
Cell-specific modulation of mitochondrial respiration and metabolism by the pro-apoptotic Bcl-2 family members Bax and Bak
Apoptosis (2024)
-
Oncogenic pathways and the electron transport chain: a dangeROS liaison
British Journal of Cancer (2020)
-
CRIF1 overexpression facilitates tumor growth and metastasis through inducing ROS/NFκB pathway in hepatocellular carcinoma
Cell Death & Disease (2020)
-
Aberrant mitochondrial function in ageing and cancer
Biogerontology (2020)
-
Loss of BIM increases mitochondrial oxygen consumption and lipid oxidation, reduces adiposity and improves insulin sensitivity in mice
Cell Death & Differentiation (2018)