Abstract
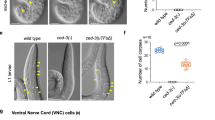
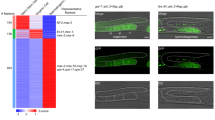
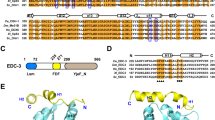
In Caenorhabditis elegans, apoptosis in germ cells is mediated by the same core apoptotic machinery that controls apoptosis in somatic cells. These include the CED-3 caspase, the CED-3 activator CED-4, and the cell death inhibitor CED-9. However, germline apoptosis also differs from somatic apoptosis in its regulation. We found that CSP-3, a caspase homolog that blocks CED-3 autoactivation and apoptosis in somatic cells, does not affect apoptosis in germ cells. Interestingly, the second C. elegans caspase homolog, CSP-2, shares sequence similarity to both catalytic subunits of the CED-3 caspase, and surprisingly, contains a stretch of sequence that is almost identical to that of CSP-3. Unlike CSP-3 that acts specifically in somatic cells, loss of CSP-2 causes increased apoptosis only in germ cells, suggesting that CSP-2 is a germ cell-specific apoptosis inhibitor. Moreover, like CSP-3, CSP-2 associates with the CED-3 zymogen and inhibits its autoactivation, but does not inhibit CED-4-induced CED-3 activation or the activity of the activated CED-3 protease. Thus, two different C. elegans caspase homologs use the same mechanism to prevent caspase autoactivation and apoptosis in different tissues, suggesting that this could be a generally applicable strategy for regulating caspase activation and apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AA:
-
amino acid
- GFP:
-
green fluorescent protein
- L1-L4:
-
larval stages 1-4
- N2:
-
wild-type C. elegans strains-Bristol isolate
- PCD:
-
programmed cell death
- RT-PCR:
-
reverse transcriptase polymerase chain reaction
- WT:
-
wild type
- CED:
-
cell death abnormal
- S.E.M:
-
standard error of the mean
References
Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO . Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1999; 126: 1011–1022.
Gartner A, Boag PR, Blackwell TK . Germline survival and apoptosis. WormBook 2008: 1–20. doi/10.1895/wormbook.1.145.1, http://www.nature.com/cdd.
Andux S, Ellis RE . Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet 2008; 4: e1000295.
Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO . A conserved checkpoint pathway mediates DNA damage – induced apoptosis and cell cycle arrest in C. elegans. Mol Cell 2000; 5: 435–443.
Shi Y . Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 2002; 9: 459–470.
Boatright KM, Salvesen GS . Mechanisms of caspase activation. Curr Opin Cell Biol 2003; 15: 725–731.
Deveraux QL, Reed JC . IAP family proteins – suppressors of apoptosis. Genes Dev 1999; 13: 239–252.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC . X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997; 388: 300–304.
Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC . The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 1997; 16: 6914–6925.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 1998; 17: 2215–2223.
Meier P, Silke J, Leevers SJ, Evan GI . The Drosophila caspase DRONC is regulated by DIAP1. EMBO J 2000; 19: 598–611.
Hawkins CJ, Wang SL, Hay BA . A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc Natl Acad Sci USA 1999; 96: 2885–2890.
Geng X, Shi Y, Nakagawa A, Yoshina S, Mitani S, Xue D . Inhibition of CED-3 zymogen activation and apoptosis in Caenorhabditis elegans by caspase homolog CSP-3. Nat Struct Mol Biol 2008; 15: 1094–1101.
Shaham S . Identification of multiple Caenorhabditis elegans caspases and their potential roles in proteolytic cascades. J Biol Chem 1998; 273: 35109–35117.
Cohen GM . Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16.
Blumenthal T . Trans-splicing and operons. WormBook 2005: 1–9.
Ellis RE, Jacobson DM, Horvitz HR . Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 1991; 129: 79–94.
Hengartner MO, Ellis RE, Horvitz HR . Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 1992; 356: 494–499.
Praitis V, Casey E, Collar D, Austin J . Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 2001; 157: 1217–1226.
Tenenhaus C, Subramaniam K, Dunn MA, Seydoux G . PIE-1 is a bifunctional protein that regulates maternal and zygotic gene expression in the embryonic germ line of Caenorhabditis elegans. Genes Dev 2001; 15: 1031–1040.
Xue D, Shaham S, Horvitz HR . The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev 1996; 10: 1073–1083.
Rotonda J, Nicholson DW, Fazil KM, Gallant M, Gareau Y, Labelle M et al. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nat Struct Biol 1996; 3: 619–625.
Sulston JE, Horvitz HR . Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 1977; 56: 110–156.
Brenner S . The genetics of Caenorhabditis elegans. Genetics 1974; 77: 71–94.
Riddle DL, Blumenthal T, Meyer BJ, Preiss JR (eds). C. elegans II. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1997.
Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A et al. Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci USA 1997; 94: 13128–13133.
Gengyo-Ando K, Mitani S . Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem Biophys Res Commun 2000; 269: 64–69.
Darland-Ransom M, Wang X, Sun CL, Mapes J, Gengyo-Ando K, Mitani S et al. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science 2008; 320: 528–531.
Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol 2007; 9: 541–549.
Yan N, Chai J, Lee ES, Gu L, Liu Q, He J et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature 2005; 437: 831–837.
Acknowledgements
We thank N Pace for advice on phylogenetic analysis, Y Shi and members of the Xue laboratory for comments and discussions, L Yang, HW Yang, and CL Sun for technical support, S Shaham for cDNA clones, and M Driscoll (Rutgers University) for the bzIs8 strain. This work was supported by NIH R01 grants (GM059083 and GM079097) and a Burroughs Welcome Fund Award to DX and a grant from MEXT of Japan to SM. XG was supported by U Colorado Matching Grant to the SCR Training Grant (T32 GM08759).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by RA Knight
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Geng, X., Zhou, Q., Kage-Nakadai, E. et al. Caenorhabditis elegans caspase homolog CSP-2 inhibits CED-3 autoactivation and apoptosis in germ cells. Cell Death Differ 16, 1385–1394 (2009). https://doi.org/10.1038/cdd.2009.88
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2009.88
Keywords
This article is cited by
-
Intertwined Functions of Separase and Caspase in Cell Division and Programmed Cell Death
Scientific Reports (2020)
-
Programmed cell death and clearance of cell corpses in Caenorhabditis elegans
Cellular and Molecular Life Sciences (2016)