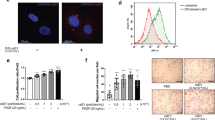
Abstract
The apoptotic program incorporates a paracrine component of importance in fostering tissue repair at sites of apoptotic cell deletion. As this paracrine pathway likely bears special importance in maladaptive intercellular communication leading to vascular remodeling, we aimed at further defining the mediators produced by apoptotic endothelial cells (EC), using comparative and functional proteomics. Apoptotic EC were found to release nanovesicles displaying ultrastructural characteristics, protein markers and functional activity that differed from apoptotic blebs. Tumor susceptibility gene 101 and translationally controlled tumor protein (TCTP) were identified in nanovesicle fractions purified from medium conditioned by apoptotic EC and absent from purified apoptotic blebs. Immunogold labeling identified TCTP on the surface of nanovesicles purified from medium conditioned by apoptotic EC and within multivesicular blebs in apoptotic EC. These nanovesicles induced an extracellular signal-regulated kinases 1/2 (ERK 1/2)-dependent antiapoptotic phenotype in vascular smooth muscle cells (VSMC), whereas apoptotic blebs did not display antiapoptotic activity on VSMC. Caspase-3 biochemical inhibition and caspase-3 RNA interference in EC submitted to a proapoptotic stimulus inhibited the release of nanovesicles. Also, TCTP siRNAs in EC attenuated the antiapoptotic activity of purified nanovesicles on VSMC. Collectively, these results identify TCTP-bearing nanovesicles as a novel component of the paracrine apoptotic program of potential importance in vascular repair.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 2D-LC-MS/MS:
-
two-dimensional liquid chromatography and tandem mass spectrometry
- CTGF:
-
connective tissue growth factor
- DMSO:
-
dimethylsulfoxide
- EC:
-
endothelial cells
- ERK 1/2:
-
extracellular signal-regulated kinases 1/2
- FPLC:
-
fast protein liquid chromatography
- HO:
-
Hoechst 33342 (2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2.5′-bi-1H-benzimidazole)
- HUVEC:
-
human umbilical vascular endothelial cells
- LDH:
-
lactate dehydrogenase
- LG3:
-
C-terminal fragment of perlecan
- MMC:
-
mitomycin C
- MVB:
-
multivesicular bodies
- PARP:
-
poly(ADP-ribose) polymerase
- PI:
-
propidium iodide
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- siRNAs:
-
small interfering RNAs
- SN:
-
exosome-free supernatant
- SS:
-
serum starvation
- SSC:
-
serum-free media conditioned by endothelial cells
- TCTP:
-
translationally-controlled tumor protein
- TSG 101:
-
tumor susceptibility gene 101
- VSMC:
-
vascular smooth muscle cells
- LC3:
-
microtubule-associated proteins 1A and 1B, light chain 3
- mTOR:
-
mammalian target of rapamycin
References
Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003; 113: 717–730.
Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest 2009; 119: 20–32.
Raymond MA, Desormeaux A, Laplante P, Vigneault N, Filep JG, Landry K et al. Apoptosis of endothelial cells triggers a caspase-dependent anti-apoptotic paracrine loop active on VSMC. Faseb J 2004; 18: 705–707.
Laplante P, Raymond MA, Gagnon G, Vigneault N, Sasseville AM, Langelier Y et al. Novel fibrogenic pathways are activated in response to endothelial apoptosis: implications in the pathophysiology of systemic sclerosis. J Immunol 2005; 174: 5740–5749.
Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hebert MJ . Perlecan proteolysis induces an alpha2beta1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem 2006; 281: 30383–30392.
Cailhier JF, Sirois I, Laplante P, Lepage S, Raymond MA, Brassard N et al. Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis. J Biol Chem 2008; 283: 27220–27229.
Soulez M, Sirois I, Brassard N, Raymond MA, Nicodeme F, Noiseux N et al. Epidermal growth factor and perlecan fragments produced by apoptotic endothelial cells co-ordinately activate ERK1/2-dependent antiapoptotic pathways in mesenchymal stem cells. Stem Cells 2010; 28: 810–820.
Laplante P, Sirois I, Raymond MA, Kokta V, Beliveau A, Prat A et al. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ 2010; 17: 291–303.
Cailhier JF, Laplante P, Hebert MJ . Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am J Transplant 2006; 6: 247–253.
Cornell LD, Smith RN, Colvin RB . Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol 2008; 3: 189–220.
Pollman MJ, Hall JL, Mann MJ, Zhang L, Gibbons GH . Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat Med 1998; 4: 222–227.
Gennaro G, Menard C, Michaud SE, Deblois D, Rivard A . Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation 2004; 110: 3367–3371.
Hirata A, Igarashi M, Yamaguchi H, Suwabe A, Daimon M, Kato T et al. Nifedipine suppresses neointimal thickening by its inhibitory effect on vascular smooth muscle cell growth via a MEK-ERK pathway coupling with Pyk2. Br J Pharmacol 2000; 131: 1521–1530.
Pshezhetsky AV, Fedjaev M, Ashmarina L, Mazur A, Budman L, Sinnett D et al. Subcellular proteomics of cell differentiation: quantitative analysis of the plasma membrane proteome of Caco-2 cells. Proteomics 2007; 7: 2201–2215.
Thery C, Ostrowski M, Segura E . Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–593.
Amzallag N, Passer BJ, Allanic D, Segura E, Thery C, Goud B et al. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem 2004; 279: 46104–46112.
Yu X, Harris SL, Levine AJ . The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006; 66: 4795–4801.
Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ 2008; 15: 1723–1733.
Yoon T, Jung J, Kim M, Lee KM, Choi EC, Lee K . Identification of the self-interaction of rat TCTP/IgE-dependent histamine-releasing factor using yeast two-hybrid system. Arch Biochem Biophys 2000; 384: 379–382.
Kim M, Min HJ, Won HY, Park H, Lee JC, Park HW et al. Dimerization of translationally controlled tumor protein is essential for its cytokine-like activity. PLoS ONE 2009; 4: e6464.
Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001; 166: 7309–7318.
Thery C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579.
Li XB, Zhang ZR, Schluesener HJ, Xu SQ . Role of exosomes in immune regulation. J Cell Mol Med 2006; 10: 364–375.
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16: 3–11.
Chen SH, Wu PS, Chou CH, Yan YT, Liu H, Weng SY et al. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific manner. Mol Biol Cell 2007; 18: 2525–2532.
Telerman A, Amson R . The molecular programme of tumour reversion: the steps beyond malignant transformation. Nat Rev Cancer 2009; 9: 206–216.
Vonakis BM, Macglashan Jr DW, Vilarino N, Langdon JM, Scott RS, MacDonald SM . Distinct characteristics of signal transduction events by histamine-releasing factor/translationally controlled tumor protein (HRF/TCTP)-induced priming and activation of human basophils. Blood 2008; 111: 1789–1796.
Paquet C, Sane AT, Beauchemin M, Bertrand R . Caspase- and mitochondrial dysfunction-dependent mechanisms of lysosomal leakage and cathepsin B activation in DNA damage-induced apoptosis. Leukemia 2005; 19: 784–791.
Takeuchi H, Kanzawa T, Kondo Y, Kondo S . Inhibition of platelet-derived growth factor signalling induces autophagy in malignant glioma cells. Br J Cancer 2004; 90: 1069–1075.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183: 1161–1172.
Bendayan M . Tech.Sight. Worth its weight in gold. Science 2001; 291: 1363–1365.
Pisitkun T, Shen RF, Knepper MA . Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 2004; 101: 13368–13373.
Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, Condon TP et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res 2005; 11: 2492–2501.
Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003; 278: 10963–10972.
Aronov S, Gelin-Licht R, Zipor G, Haim L, Safran E, Gerst JE . mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol Cell Biol 2007; 27: 3441–3455.
Nguyen NV, Gleeson PA, Courtois-Coutry N, Caplan MJ, Van Driel IR . Gastric parietal cell acid secretion in mice can be regulated independently of H/K ATPase endocytosis. Gastroenterology 2004; 127: 145–154.
Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC et al. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J Biol Chem 2004; 279: 15434–15440.
Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogen CM, de Priester W, Westmuckett A, Lupu F . An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol 1997; 139: 245–256.
van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001; 121: 337–349.
Segura E, Amigorena S, Thery C . Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis 2005; 35: 89–93.
Acknowledgements
This work was supported by research grants from the Canadian Institutes of Health Research to MJH (MOP-15447) and the Canadian Institutes of Health Research to AVP (MOP-66980). MJH is the holder of the Shire Chair in Nephrology, Transplantation and Renal Regeneration of the University of Montreal. IS is the recipient of a training fellowship from the Canadian Institutes of Health Research. JFC is an FRSQ scholar. We thank the J-L Lévesque Foundation for renewed support, Nicolas Parent for help with JC-1 staining assay and D. Gingras for processing cells for electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Vandenabeele
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Sirois, I., Raymond, MA., Brassard, N. et al. Caspase-3-dependent export of TCTP: a novel pathway for antiapoptotic intercellular communication. Cell Death Differ 18, 549–562 (2011). https://doi.org/10.1038/cdd.2010.126
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.126
Keywords
This article is cited by
-
Advances in biological functions and applications of apoptotic vesicles
Cell Communication and Signaling (2023)
-
Apoptotic exosome-like vesicles transfer specific and functional mRNAs to endothelial cells by phosphatidylserine-dependent macropinocytosis
Cell Death & Disease (2023)
-
Apoptotic exosome-like vesicles regulate endothelial gene expression, inflammatory signaling, and function through the NF-κB signaling pathway
Scientific Reports (2020)
-
Apoptotic cell-derived exosomes: messages from dying cells
Experimental & Molecular Medicine (2020)
-
Molecular isolation and characterization of translationally controlled tumor protein (TCTP) gene from Macrobrachium rosenbergii
Aquaculture International (2020)