Abstract
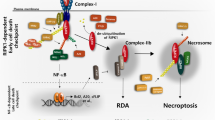
Three members of the IAP family (X-linked inhibitor of apoptosis (XIAP), cellular inhibitor of apoptosis proteins-1/-2 (cIAP1 and cIAP2)) are potent suppressors of apoptosis. Recent studies have shown that cIAP1 and cIAP2, unlike XIAP, are not direct caspase inhibitors, but block apoptosis by functioning as E3 ligases for effector caspases and receptor-interacting protein 1 (RIP1). cIAP-mediated polyubiquitination of RIP1 allows it to bind to the pro-survival kinase transforming growth factor-β-activated kinase 1 (TAK1) which prevents it from activating caspase-8-dependent death, a process reverted by the de-ubiquitinase CYLD. RIP1 is also a regulator of necrosis, a caspase-independent type of cell death. Here, we show that cells depleted of the IAPs by treatment with the IAP antagonist BV6 are greatly sensitized to tumor necrosis factor (TNF)-induced necrosis, but not to necrotic death induced by anti-Fas, poly(I:C) oxidative stress. Specific targeting of the IAPs by RNAi revealed that repression of cIAP1 is responsible for the sensitization. Similarly, lowering TAK1 levels or inhibiting its kinase activity sensitized cells to TNF-induced necrosis, whereas repressing CYLD had the opposite effect. We show that this sensitization to death is accompanied by enhanced RIP1 kinase activity, increased recruitment of RIP1 to Fas-associated via death domain and RIP3 (which allows necrosome formation), and elevated RIP1 kinase-dependent accumulation of reactive oxygen species (ROS). In conclusion, our data indicate that cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent ROS production.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 5Z-7:
-
5Z-7-oxozeaenol
- BHA:
-
butylated hydroxyanisole
- cIAP1/2:
-
cellular inhibitor of apoptosis proteins-1/-2
- CYLD:
-
cylindromatosis
- DHR123:
-
dihydrorhodamine 123
- ERK:
-
extracellular signal-regulated kinase
- FADD:
-
Fas-associated via death domain
- H2O2:
-
hydrogen peroxide
- IFNβ:
-
interferon-β
- MEK1/2:
-
mitogen-activated protein kinase kinase 1/-2
- NDUFB8:
-
NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8
- Nec-1:
-
necrostatin-1
- Nox1:
-
NADPH oxidase 1
- RFK:
-
riboflavin kinase
- RIP:
-
receptor-interacting protein
- ROS:
-
reactive oxygen species
- TAK1:
-
transforming growth factor-β-activated kinase 1
- TLR3:
-
toll-like receptor 3
- TNF:
-
tumor necrosis factor
- TNFR1:
-
tumor necrosis factor receptor 1
- XIAP:
-
X-linked inhibitor of apoptosis
- zVAD-fmk:
-
benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
References
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G . Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11: 700–714.
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 1998; 187: 1477–1485.
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–321.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119.
Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 2010; 17: 922–930.
Vanlangenakker N, Berghe TV, Krysko DV, Festjens N, Vandenabeele P . Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med 2008; 8: 207–220.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–336.
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al. Receptor interacting protein kinase-3 (RIP3) determines cellular necrotic response to TNFa. Cell 2009; 137: 1100–1111.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000; 1: 489–495.
Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al. Phosphorylation-driven assembly of RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–1123.
Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P . RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ 2007; 14: 400–410.
Eckelman BP, Salvesen GS . The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 2006; 281: 3254–3260.
Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM, Bratton SB . The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspases-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem 2009; 284: 12772–12782.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 2008; 30: 689–700.
Komander D . The emerging complexity of protein ubiquitination. Biochem Soc Trans 2009; 37: 937–953.
Wang L, Du F, Wang X . TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703.
Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 1998; 188: 919–930.
Kalai M, Van Loo G, Vanden Berghe T, Meeus A, Burm W, Saelens X et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ 2002; 9: 981–994.
Varfolomeev E, Alicke B, Elliott JM, Zobel K, West K, Wong H et al. XIAP regulates cell death induction by pro-apoptotic receptor agonists. J Biol Chem 2009; 284: 34553–34560.
Holcik M, Lefebvre CA, Hicks K, Korneluk RG . Cloning and characterization of the rat homologues of the inhibitor of apoptosis protein 1, 2, and 3 genes. BMC Genom 2002; 3: 5.
Devin A, Lin Y, Liu ZG . The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep 2003; 4: 623–627.
Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem 2004; 279: 10822–10828.
Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W . Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem 1992; 267: 5317–5323.
Kim YS, Morgan MJ, Choksi S, Liu ZG . TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 2007; 26: 675–687.
Festjens N, Kalai M, Smet J, Meeus A, Van Coster R, Saelens X et al. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ 2006; 13: 166–169.
Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 2009; 460: 1159–1163.
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16: 3–11.
Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem 2008; 283: 24295–24299.
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA 2008; 105: 11778–11783.
Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol 2009; 187: 1037–1054.
Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Hacker G . Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ 2010; 17: 942–951.
Friboulet L, Pioche-Durieu C, Rodriguez S, Valent A, Souquere S, Ripoche H et al. Recurrent overexpression of c-IAP2 in EBV-associated nasopharyngeal carcinomas: critical role in resistance to Toll-like receptor 3-mediated apoptosis. Neoplasia 2008; 10: 1183–1194.
Chang M, Jin W, Sun SC . Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol 2009; 10: 1089–1095.
Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J . RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ 2010; 17: 482–487.
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P . The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998; 8: 297–303.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ . TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001; 412: 346–351.
Park SM, Yoon JB, Lee TH . Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett 2004; 566: 151–156.
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M . Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120: 649–661.
Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P . Necrosis is associated with IL-6 production but apoptosis is not. Cell Signal 2006; 18: 328–335.
Acknowledgements
We thank Professor Wim Declercq and Dr. Saskia Lippens for critical feedback and discussion and Dr. A Bredan for editing. We are grateful to Dr. RG Korneluk for sending the RIAP1 antibody. TV and MB received a postdoctoral fellowship from the FWO, PB is paid by VIB, and NV obtained a predoctoral fellowship from the BOF, Ghent University. BL was a master student working in the labs of both Professor Simone Fulda and Professor Peter Vandenabeele. Research in the Vandenabeele group is supported by VIB, Ghent University, Research Foundation Flanders (FWO-Vlaanderen) (3G.0218.06 and G.0226.09), Federal Research Program IAP 6/18, European Research Program FP6 ApopTrain (MRTN-CT-035624) and FP7 Apo-Sys 200767, and the GROUP-ID consortium of the UGent MRP initiative. PV holds a Methusalem Grant (BOF09/01M00709) from the Flemish Government. Research in the Fulda group is supported by the Deutsche Forschungsgemeinschaft, Federal Research Program IAP 6/18, European Research Program FP6 ApopTrain (MRTN-CT-035624) and FP7 Apo-Sys 200767.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Deshmukh
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Vanlangenakker, N., Vanden Berghe, T., Bogaert, P. et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ 18, 656–665 (2011). https://doi.org/10.1038/cdd.2010.138
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.138
Keywords
This article is cited by
-
Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review
Cellular & Molecular Biology Letters (2023)
-
The development of necroptosis: what we can learn
Cell Stress and Chaperones (2023)
-
Role of necroptosis in airflow limitation in chronic obstructive pulmonary disease: focus on small-airway disease and emphysema
Cell Death Discovery (2022)
-
Aluminum Induced Necroptosis of PC12 Cells via TNFR1-RIP1/RIP3 Signalling Pathway
Neurochemical Research (2022)
-
Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor
Cell Death & Disease (2021)