Abstract
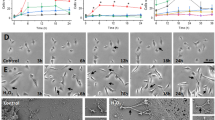
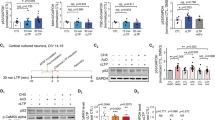
Tunneling nanotubes (TNTs) can be induced in rat hippocampal astrocytes and neurons with H2O2 or serum depletion. Major cytoskeletal component of TNTs is F-actin. TNTs transfer endoplasmic reticulum, mitochondria, Golgi, endosome and intracellular as well as extracellular amyloid β. TNT development is a property of cells under stress. When two populations of cells are co-cultured, it is the stressed cells that always develop TNTs toward the unstressed cells. p53 is crucial for TNT development. When p53 function is deleted by either dominant negative construct or siRNAs, TNT development is inhibited. In addition, we find that among the genes activated by p53, epidermal growth factor receptor is also important to TNT development. Akt, phosphoinositide 3-kinase and mTOR are involved in TNT induction. Our data suggest that TNTs might be a mechanism for cells to respond to harmful signals and transfer cellular substances or energy to another cell under stress.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Aβ:
-
amyloid β
- ANOVA:
-
analysis of variance
- BCA:
-
bicinchoninic acid
- CBX:
-
carbenoxolone
- EGF:
-
epidermal growth factor
- EGFR:
-
epidermal growth factor receptor
- ER:
-
endoplasmic reticulum
- FBS:
-
fetal bovine serum
- HIV:
-
human immunodeficiency virus
- HRP:
-
horseradish peroxidase
- NGF:
-
nerve growth factor
- PBS:
-
phosphate-buffered saline
- PI3K:
-
phosphoinositide 3-kinase
- TBS:
-
Tris-buffered saline
- TBS-T:
-
Tris-buffered saline with tween20
- TC-Aβ:
-
tetracysteine-amyloid β
- TGF-α:
-
transforming growth factor-α
- TNT:
-
tunneling nanotube
- TUNEL:
-
terminal deoxynucleotidyl transferase-biotin dUTP nick-end labeling
References
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH . Nanotubular highways for intercellular organelle transport. Science 2004; 303: 1007–1010.
Onfelt B, Davis DM . Can membrane nanotubes facilitate communication between immune cells? Biochem Soc Trans 2004; 32 (Part 5): 676–678.
Onfelt B, Nedvetzki S, Yanagi K, Davis DM . Cutting edge: membrane nanotubes connect immune cells. J Immunol 2004; 173: 1511–1513.
Vidulescu C, Clejan S, O’Connor KC . Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med 2004; 8: 388–396.
Watkins SC, Salter RD . Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 2005; 23: 309–318.
Wustner D . Plasma membrane sterol distribution resembles the surface topography of living cells. Mol Biol Cell 2007; 18: 211–228.
Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol 2006; 177: 8476–8483.
Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC . Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci 2005; 118 (Part 16): 3695–3703.
Freund D, Bauer N, Boxberger S, Feldmann S, Streller U, Ehninger G et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev 2006; 15: 815–829.
Ramirez-Weber FA, Kornberg TB . Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 1999; 97: 599–607.
Gurke S, Barroso JF, Gerdes HH . The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol 2008; 129: 539–550.
Gerdes HH, Bukoreshtliev NV, Barroso JF . Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett 2007; 581: 2194–2201.
Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 2009; 11: 328–336.
Gousset K, Zurzolo C . Tunnelling nanotubes: a highway for prion spreading? Prion 2009; 3: 94–98.
Davis DM, Sowinski S . Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol 2008; 9: 431–436.
Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 2008; 10: 211–219.
Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN . Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem 2002; 277: 15666–15670.
Grant SM, Shankar SL, Chalmers-Redman RM, Tatton WG, Szyf M, Cuello AC . Mitochondrial abnormalities in neuroectodermal cells stably expressing human amyloid precursor protein (hAPP751). Neuroreport 1999; 10: 41.
Zhang Y, McLaughlin R, Goodyer C, LeBlanc A . Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol 2002; 156: 519–529.
Zhang Y, Goodyer C, LeBlanc A . Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci 2000; 20: 8384–8389.
Riley T, Sontag E, Chen P, Levine A . Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 2008; 9: 402–412.
Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW . Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003; 284: 31–53.
Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol 2009; 11: 1427–1432.
Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH . Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res 2008; 314: 3669–3683.
Cui J, Chen Q, Yue X, Jiang X, Gao GF, Yu LC et al. Galanin protects against intracellular amyloid toxicity in human primary neurons. J Alzheimers Dis 2010; 19: 529–544.
Morris LG, Veeriah S, Chan TA . Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene 2010; 29: 3453–3464.
Gadea G, Lapasset L, Gauthier-Rouviere C, Roux P . Regulation of Cdc42-mediated morphological effects: a novel function for p53. EMBO J 2002; 21: 2373–2382.
Guenal I, Risler Y, Mignotte B . Down-regulation of actin genes precedes microfilament network disruption and actin cleavage during p53-mediated apoptosis. J Cell Sci 1997; 110 (Part 4): 489–495.
Comer KA, Dennis PA, Armstrong L, Catino JJ, Kastan MB, Kumar CC . Human smooth muscle alpha-actin gene is a transcriptional target of the p53 tumor suppressor protein. Oncogene 1998; 16: 1299–1308.
Metcalfe S, Weeds A, Okorokov AL, Milner J, Cockman M, Pope B . Wild-type p53 protein shows calcium-dependent binding to F-actin. Oncogene 1999; 18: 2351–2355.
Xie W, Paterson AJ, Chin E, Nabell LM, Kudlow JE . Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol Endocrinol 1997; 11: 1766–1781.
Vassar R, Fuchs E . Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev 1991; 5: 714–727.
Dominey AM, Wang XJ, King Jr LE, Nanney LB, Gagne TA, Sellheyer K et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ 1993; 4: 1071–1082.
Blum M . A null mutation in TGF-alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nat Neurosci 1998; 1: 374–377.
Averous J, Proud CG . When translation meets transformation: the mTOR story. Oncogene 2006; 25: 6423–6435.
Wang X, Proud CG . The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006; 21: 362–369.
Hinds PW, Finlay CA, Quartin RS, Baker SJ, Fearon ER, Vogelstein B et al. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the ‘hot spot’ mutant phenotypes. Cell Growth Differ 1990; 1: 571–580.
Coffer PJ, Woodgett JR . Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem 1991; 201: 475–481.
Zhang Y, Hong Y, Bounhar Y, Blacker M, Roucou X, Tounekti O et al. p75 neurotrophin receptor protects primary cultures of human neurons against extracellular amyloid beta peptide cytotoxicity. J Neurosci 2003; 23: 7385–7394.
Acknowledgements
We thank Dr. A Levine (Rockefeller University) for providing wild-type and dominant negative mutant p53 constructs, Dr. J Woodgett (Ontario Cancer Institute) for providing wild-type, dominate negative and constitutively active mutant Akt constructs and Dr. H Lashuel (EPFL) for providing TC-Aβ. We also thank the assistance of confocal imaging from Dr. Shiqiang Wang and Mr. Rongchang Li (Peking University). This work was supported by the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2009CB941301), Peking University President Research Grant and Ministry of Education Recruiting Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by N Bazan
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Y., Cui, J., Sun, X. et al. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ 18, 732–742 (2011). https://doi.org/10.1038/cdd.2010.147
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.147
Keywords
This article is cited by
-
ROS/mtROS promotes TNTs formation via the PI3K/AKT/mTOR pathway to protect against mitochondrial damages in glial cells induced by engineered nanomaterials
Particle and Fibre Toxicology (2024)
-
Rescue of mitochondrial import failure by intercellular organellar transfer
Nature Communications (2024)
-
Mitochondrial transfer in hematological malignancies
Biomarker Research (2023)
-
Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria
Cell Death & Disease (2023)
-
Rescuers from the Other Shore: Intercellular Mitochondrial Transfer and Its Implications in Central Nervous System Injury and Diseases
Cellular and Molecular Neurobiology (2023)