Abstract
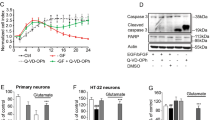
In vitro stem cell systems traditionally employ oxygen levels that are far removed from the in vivo situation. This study investigates whether an ambient environment containing a physiological oxygen level of 3% (normoxia) enables the generation of neural precursor cells (NPCs) from human embryonic stem cells (hESCs) and whether the resultant NPCs can undergo regional specification and functional maturation. We report robust and efficient neural conversion at 3% O2, demonstration of tri-lineage potential of resultant NPCs and the subsequent electrophysiological maturation of neurons. We also show that NPCs derived under 3% O2 can be differentiated long term in the absence of neurotrophins and can be readily specified into both spinal motor neurons and midbrain dopaminergic neurons. Finally, modelling the oxygen stress that occurs during transplantation, we demonstrate that in vitro transfer of NPCs from a 20 to 3% O2 environment results in significant cell death, while maintenance in 3% O2 is protective. Together these findings support 3% O2 as a physiologically relevant system to study stem cell-derived neuronal differentiation and function as well as to model neuronal injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BDNF:
-
brain derived neurotrophic factor
- CDM:
-
chemically defined medium
- CNS:
-
central nervous system
- FGF-2:
-
fibroblast growth factor-2
- GDNF:
-
glial derived neurotrophic factor
- hESCs:
-
human embryonic stem cells
- hESC-NPCs:
-
human embryonic stem cell derived neural precursor cells
- HIF:
-
hypoxia inducible factor
- IGF:
-
insulin-like growth factor
- MBP:
-
myelin basic protein
- NAC:
-
N-acetyl-l-cysteine
- NPC:
-
neural precursor cell
- NSC:
-
neural stem cell
- O2:
-
oxygen
- pO2:
-
partial pressure of oxygen
- RA:
-
retinoic acid
- ROS:
-
reactive oxygen species
- TH:
-
tyrosine hydroxylase
- TTX:
-
tetrodotoxin
- VMAT:
-
vesicular monoamine transporter
References
Zhao T, Zhang CP, Liu ZH, Wu LY, Huang X, Wu HT et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells—role of hypoxia-inducible transcription factor-1alpha. FEBS J 2008; 275: 1824–1834.
Erceg S, Ronaghi M, Stojkovic M . Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells 2009; 27: 78–87.
Hedlund E, Perlmann T . Neuronal cell replacement in Parkinson's disease. J Intern Med 2009; 266: 358–371.
Conti L, Cattaneo E . Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci 2010; 11: 176–187.
Munoz-Sanjuan I, Brivanlou AH . Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 2002; 3: 271–280.
Smukler SR, Runciman SB, Xu S, van der KD . Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol 2006; 172: 79–90.
Clarke L, van der KD . Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells 2009; 27: 1879–1886.
Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM . Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells 2007; 25: 2291–2301.
Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM . Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 2007; 35: 424–435.
Cimadamore F, Curchoe CL, Alderson N, Scott F, Salvesen G, Terskikh AV . Nicotinamide rescues human embryonic stem cell-derived neuroectoderm from parthanatic cell death. Stem Cells 2009; 27: 1772–1781.
Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 2004; 101: 12543–12548.
Li TS, Marban E . Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 2010; 28: 1178–1185.
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L . Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009; 27: 275–280.
Patani R, Compston A, Puddifoot CA, Wyllie DJ, Hardingham GE, Allen ND et al. Activin/nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS One 2009; 4: e7327.
Erecinska M, Silver IA . Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 2001; 128: 263–276.
Dings J, Meixensberger J, Jager A, Roosen K . Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998; 43: 1082–1095.
Csete M . Oxygen in the cultivation of stem cells. Ann N Y Acad Sci 2005; 1049: 1–8.
Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 2000; 20: 7377–7383.
Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD . Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2009; 139: 85–97.
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S . Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009; 5: 237–241.
Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 2005; 9: 617–628.
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006; 20: 557–570.
Akundi RS, Rivkees SA . Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PLoS One 2009; 4: e4739.
Simon MC, Keith B . The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 2008; 9: 285–296.
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD . Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 2007; 104: 5431–5436.
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010; 7: 380–390.
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A . Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010; 7: 150–161.
Li D, Marks JD, Schumacker PT, Young RM, Brorson JR . Physiological hypoxia promotes survival of cultured cortical neurons. Eur J Neurosci 2005; 22: 1319–1326.
Trotti D, Danbolt NC, Volterra A . Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 1998; 19: 328–334.
Behl C, Moosmann B . Oxidative nerve cell death in Alzheimer's disease and stroke: antioxidants as neuroprotective compounds. Biol Chem 2002; 383: 521–536.
Johansson BM, Wiles MV . Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol 1995; 15: 141–151.
Vallier L, Alexander M, Pedersen RA . Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005; 118 (Part 19): 4495–4509.
Joannides AJ, Fiore-Heriche C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 2007; 25: 731–737.
Arteel GE, Thurman RG, Yates JM, Raleigh JA . Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 1995; 72: 889–895.
Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 2005; 23: 781–790.
Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 2008; 26: 886–893.
Semenza GL . Life with oxygen. Science 2007; 318: 62–64.
Moe MC, Varghese M, Danilov AI, Westerlund U, Ramm-Pettersen J, Brundin L et al. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain 2005; 128: 2189–2199.
Hanson Jr MG, Shen S, Wiemelt AP, McMorris FA, Barres BA . Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro . J Neurosci 1998; 18: 7361–7371.
Cameron CM, Harding F, Hu WS, Kaufman DS . Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2008; 233: 1044–1057.
Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D, Keith B et al. HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci USA 2009; 106: 14391–14396.
Rosova I, Dao M, Capoccia B, Link D, Nolta JA . Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008; 26: 2173–2182.
Ivanovic Z . Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol 2009; 219: 271–275.
Acknowledgements
We thank James Raleigh for his generous gift of pimonidazole (Hypoxyprobe) and antibody, Ludovic Vallier for his kind provision of feeder-free H9 ES cultures, Roger Barker for use of the low oxygen incubator and Rickie Patani, Kristine Westmore and David Story for valuable technical assistance. This work was supported by the MS Society UK, Evelyn Trust, MRC, Wellcome Trust (AL) and Royal Society (RK). SS is supported by a Sir David Walker Fellowship, a joint Medical Research Council and Multiple Sclerosis Society Clinical Research Training Fellowship (no. G0800487) and a Raymond and Beverly Sackler Studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Verkhratsky
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Stacpoole, S., Bilican, B., Webber, D. et al. Derivation of neural precursor cells from human ES cells at 3% O2 is efficient, enhances survival and presents no barrier to regional specification and functional differentiation. Cell Death Differ 18, 1016–1023 (2011). https://doi.org/10.1038/cdd.2010.171
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.171
Keywords
This article is cited by
-
Nup133 and ERα mediate the differential effects of hyperoxia-induced damage in male and female OPCs
Molecular and Cellular Pediatrics (2020)
-
Thyroid-related hormones as potential markers of hypoxia/ischemia
Human Cell (2020)
-
Cellular regeneration strategies for macular degeneration: past, present and future
Eye (2018)
-
Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen
Nature Protocols (2011)