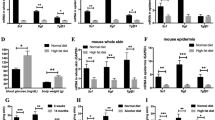
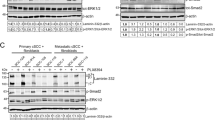
Abstract
Dysregulated reactive oxygen species (ROS) generation contributes to many human pathologies, including cancer and diabetes. During normal wound repair, inflammation-induced ROS production must be tightly controlled, but the mechanisms reining their generation remain unclear. Herein, we show that transforming growth factor β-activated kinase 1 (TAK1) directly regulates stem cell factor (SCF) expression, which activates the protein kinase B (PKB)α pro-survival pathway in a cell-autonomous manner to protect keratinocytes from ROS-mediated cell death. TAK1 is a pivotal inflammatory mediator whose expression was transiently elevated during wound healing, paralleling the ROS production profile. TAK1 deficiency in keratinocytes led to increased apoptosis in response to anoikis and TNF-α treatment and was associated with elevated ROS level as analyzed by FACS. Using organotypic skin co-culture and comparative growth factor array analysis, we revealed a cell-autonomous mechanism that involved the SCF/c-Kit/PKBα signaling cascade. Ectopic expression of TAK1 or treatment with exogenous recombinant SCF restored the increased ROS production and apoptotic cell death in TAK1-deficient keratinocytes. Conversely, normal keratinocytes treated with various inhibitors targeting the SCF/c-Kit/PKBα pathway exhibited increased ROS production and TNF-α- or anoikis-induced apoptosis. Our study reveals a novel anti-apoptotic role for SCF in keratinocytes and identifies TAK1 as a novel player uniting inflammation and ROS regulation in skin redox biology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AP-1:
-
activating protein-1
- Col:
-
collagen
- ChIP:
-
chromatin immunoprecipitation
- DAPI:
-
4’,6-diamidino-2-phenylindole
- DCF:
-
dichlorodihydrofluorescein (CM-H2DCFDA)
- EMSA:
-
electrophoretic mobility shift assay
- FADD:
-
Fas associated death domain
- FLIP:
-
FADD-like IL-1β-converting enzyme)-inhibitory protein
- GSK:
-
glycogen synthase kinase
- H&E:
-
hematoxylin and eosin staining
- IκBα:
-
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- IKKβ:
-
inhibitor of nuclear factor kappa B kinase beta
- JNK:
-
c-Jun N-terminal kinase
- KCTRL:
-
keratinocyte control
- KTAK1:
-
keratinocyte with TAK1 knockdown
- NAC:
-
N-acetylcysteine
- OTC:
-
organotypic skin co-culture
- PCNA:
-
proliferating cell nuclear antigen
- PDGF:
-
platelet derived growth factor
- PI3K:
-
phosphoinositide-3 kinase
- PKBα:
-
protein kinase B α
- ROS:
-
reactive oxygen species
- SCF:
-
stem cell factor
- siRNA:
-
small interfering ribonucleic acid
- TAK1:
-
transforming growth factor β-activated kinase 1
- TGF-β:
-
transforming growth factor-β
- TNF-α:
-
tumor necrosis factor-α
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Albanesi C, Pastore S . Pathobiology of chronic inflammatory skin diseases: interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr Drug Metab 2010; 11: 210–227.
Pierce GF . Inflammation in nonhealing diabetic wounds: the space–time continuum does matter. Am J Pathol 2001; 159: 399–403.
Circu ML, Aw TY . Reactive oxygen species, cellular redox systems, apoptosis. Free Radic Biol Med 2010; 48: 749–762.
Roy S, Khanna S, Nallu K, Hunt TK, Sen CK . Dermal wound healing is subject to redox control. Mol Ther 2006; 13: 211–220.
D’Autréaux B, Toledano MB . ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007; 8: 813–824.
Novo E, Parola M . Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 2008; 1: 5.
Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem 2006; 281: 19610–19617.
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005; 6: 1087–1095.
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K . The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999; 398: 252–256.
Tan SH, Pal M, Tan MJ, Wong MHL, Tam FU, Teo JWT et al. Regulation of cell proliferation and migration by TAK1 via transcriptional control of von Hippel-Lindau tumor suppressor. J Biol Chem 2009; 284: 18047–18058.
Chong HC, Tan MJ, Philippe V, Tan SH, Tan CK, Ku CW et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol 2009; 184: 817–831.
Page A, Navarro M, Garín M, Pérez P, Casanova ML, Moreno R et al. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J Invest Dermatol 2010; 130: 1598–1610.
Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 2002; 417: 861–866.
Sayama K, Hanakawa Y, Nagai H, Shirakata Y, Dai X, Hirakawa S et al. Transforming growth factor-beta-activated kinase 1 is essential for differentiation and the prevention of apoptosis in epidermis. J Biol Chem 2006; 281: 22013–22020.
Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J . TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem 2008; 283: 26161–26168.
Carroll JM, Albers KM, Garlick JA, Harrington R, Taichman LB . Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc Natl Acad Sci USA 1993; 90: 10270–10274.
Thrash BR, Menges CW, Pierce RH, McCance DJ . AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem 2006; 281: 12155–12162.
Lev S, Givol D, Yarden Y . Interkinase domain of kit contains the binding site for phosphatidylinositol 3′ kinase. Proc Natl Acad Sci USA 1992; 89: 678–682.
Blume-Jensen P, Janknecht R, Hunter T . The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol 1998; 8: 779–782.
Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B . Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 2002; 10: 721–733.
Taylor WE, Najmabadi H, Strathearn M, Jou NT, Liebling M, Rajavashisth T et al. Human stem cell factor promoter deoxyribonucleic acid sequence and regulation by cyclic 3′,5′-adenosine monophosphate in a Sertoli cell line. Endocrinology 1996; 137: 5407–5414.
Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J . TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem 2008; 283: 26161–26168.
Kamata H, Honda SI, Maeda S, Chang L, Hirata H, Karin M . Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120: 649–661.
Brownlee M . Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820.
Finkel T, Serrano M, Blasco MA . The common biology of cancer and ageing. Nature 2007; 448: 767–774.
Nathan C . Points of control in inflammation. Nature 2002; 420: 846–852.
Morioka S, Omori E, Kajino T, Kajino-Sakamoto R, Matsumoto K, Ninomiya-Tsuji J . TAK1 kinase determines TRAIL sensitivity by modulating reactive oxygen species and cIAP. Cell Death Differ 2009; 28: 2257–2265.
Ashman LK . The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol 1999; 31: 1037–1051.
Grabbe J, Welker P, Rosenbach T, Nürnberg W, Krüger-Krasagakes S, Artuc M et al. Release of stem cell factor from a human keratinocyte line, HaCaT, is increased in differentiating versus proliferating cells. J Invest Dermatol 1996; 107: 219–224.
Morita E, Lee DG, Sugiyama M, Yamamoto S . Expression of c-kit ligand in human keratinocytes. Arch Dermatol Res 1994; 286: 273–277.
Peters EMJ, Maurer M, Botchkarev VA, Jensen KD, Welker P, Scott GA et al. Kit is expressed by epithelial cells in vivo. J Invest Dermatol 2003; 121: 976–984.
Shilov VN, Sergienko VI . Oxidative stress in keratinocytes as an etiopathogenetic factor of psoriasis. Bull Exp Biol Med 2000; 129: 309–313.
Basset-Séguin N, Escot C, Molès JP, Blanchard JM, Kerai C, Guilhou JJ . C-fos and c-jun proto-oncogene expression is decreased in psoriasis: an in situ quantitative analysis. J Invest Dermatol 1991; 97: 672–678.
Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, Cancino-Gómez FG, León-Dorantes G, Blancas-González F et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol 2007; 46: 239–246.
Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 2005; 437: 369–375.
Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol 2004; 2: E7.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Liou GY, Storz P . Reactive oxygen species in cancer. Free Radic Res 2010; 44: 479–496.
IJpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J 2004; 23: 2083–2091.
Acknowledgements
This work was supported by grants from Ministry of Education, Singapore (ARC 8/06) and Nanyang Technological University, Singapore (RGD 127/05, 158/06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Vandenabeele
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Lam, C., Tan, M., Tan, S. et al. TAK1 regulates SCF expression to modulate PKBα activity that protects keratinocytes from ROS-induced apoptosis. Cell Death Differ 18, 1120–1129 (2011). https://doi.org/10.1038/cdd.2010.182
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2010.182
Keywords
This article is cited by
-
Multifaceted roles of TAK1 signaling in cancer
Oncogene (2020)
-
Skin wound closure delay in metabolic syndrome correlates with SCF deficiency in keratinocytes
Scientific Reports (2020)
-
Deficiency in fibroblast PPARβ/δ reduces nonmelanoma skin cancers in mice
Cell Death & Differentiation (2020)
-
Mesenchymal stem cells restore the sperm motility from testicular torsion-detorsion injury by regulation of glucose metabolism in sperm
Stem Cell Research & Therapy (2019)
-
Angiopoietin-like 4 Mediates Colonic Inflammation by Regulating Chemokine Transcript Stability via Tristetraprolin
Scientific Reports (2017)