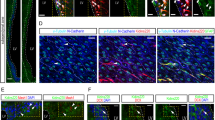
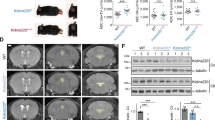
Abstract
Signaling downstream of receptor tyrosine kinases controls cell differentiation and survival. How signals from different receptors are integrated is, however, still poorly understood. In this work, we have identified Kidins220 (Kinase D interacting substrate of 220 kDa)/ARMS (Ankyrin repeat-rich membrane spanning) as a main player in the modulation of neurotrophin and vascular endothelial growth factor (VEGF) signaling in vivo, and a primary determinant for neuronal and cardiovascular development. Kidins220−/− embryos die at late stages of gestation, and show extensive cell death in the central and peripheral nervous systems. Primary neurons from Kidins220−/− mice exhibit reduced responsiveness to brain-derived neurotrophic factor, in terms of activation of mitogen-activated protein kinase signaling, neurite outgrowth and potentiation of excitatory postsynaptic currents. In addition, mice lacking Kidins220 display striking cardiovascular abnormalities, possibly due to impaired VEGF signaling. In support of this hypothesis, we demonstrate that Kidins220 constitutively interacts with VEGFR2. These findings, together with the data presented in the accompanying paper, indicate that Kidins220 mediates the integration of several growth factor receptor pathways during development, and mediates the activation of distinct downstream cascades according to the location and timing of stimulation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- CNS:
-
central nervous system
- DIV:
-
days in vitro
- DRG:
-
dorsal root ganglia
- EPSC:
-
excitatory postsynaptic current
- ES:
-
embryonic stem
- Kidins220/ARMS:
-
kinase D interacting substrate of 220 kDa/ankyrin repeat-rich membrane spanning
- MAPK/Erk:
-
mitogen-activated protein kinase/extracellular signal-activated kinase
- MN:
-
motor neuron
- NGF:
-
nerve growth factor
- Nrp1:
-
neuropilin-1
- NT:
-
neurotrophin
- p75NTR:
-
p75 neurotrophin receptor
- PNS:
-
peripheral nervous system
- Trk:
-
tropomyosin-related kinase receptor
- VEGF:
-
vascular endothelial growth factor
- VEGFR:
-
vascular endothelial growth factor receptor
References
Huang EJ, Reichardt LF . Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001; 24: 677–736.
Paratcha G, Ledda F . GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci 2008; 31: 384–391.
Lu B, Pang PT, Woo NH . The yin and yang of neurotrophin action. Nat Rev Neurosci 2005; 6: 603–614.
Snider WD . Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 1994; 77: 627–638.
Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993; 75: 113–122.
Alcantara S, Frisen J, del Rio JA, Soriano E, Barbacid M, Silos-Santiago I . TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J Neurosci 1997; 17: 3623–3633.
Bibel M, Barde YA . Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 2000; 14: 2919–2937.
Ernfors P, Van De Water T, Loring J, Jaenisch R . Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 1995; 14: 1153–1164.
Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Klein R et al. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development 1995; 121: 3381–3391.
Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994; 76: 1001–1011.
Caporali A, Emanueli C . Cardiovascular actions of neurotrophins. Physiol Rev 2009; 89: 279–308.
Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, Schiavo G . Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem 2000; 275: 40048–40056.
Kong H, Boulter J, Weber JL, Lai C, Chao MV . An evolutionarily conserved transmembrane protein that is a novel downstream target of neurotrophin and ephrin receptors. J Neurosci 2001; 21: 176–185.
Arevalo JC, Yano H, Teng KK, Chao MV . A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J 2004; 23: 2358–2368.
Neubrand VE, Thomas C, Schmidt S, Debant A, Schiavo G . Kidins220/ARMS regulates Rac1-dependent neurite outgrowth by direct interaction with the RhoGEF Trio. J Cell Sci 2010; 123: 2111–2123.
Lopez-Menendez C, Gascon S, Sobrado M, Vidaurre OG, Higuero AM, Rodriguez-Pena A et al. Kidins220/ARMS downregulation by excitotoxic activation of NMDARs reveals its involvement in neuronal survival and death pathways. J Cell Sci 2009; 122: 3554–3565.
Arevalo JC, Wu SH, Takahashi T, Zhang H, Yu T, Yano H et al. The ARMS/Kidins220 scaffold protein modulates synaptic transmission. Mol Cell Neurosci 2010; 45: 92–100.
Luo S, Chen Y, Lai KO, Arevalo JC, Froehner SC, Adams ME et al. {alpha}-Syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA4 signaling in an ARMS-dependent manner. J Cell Biol 2005; 169: 813–824.
Sniderhan LF, Stout A, Lu Y, Chao MV, Maggirwar SB . Ankyrin-rich membrane spanning protein plays a critical role in nuclear factor-kappa B signaling. Mol Cell Neurosci 2008; 38: 404–416.
Cortes RY, Arevalo JC, Magby JP, Chao MV, Plummer MR . Developmental and activity-dependent regulation of ARMS/Kidins220 in cultured rat hippocampal neurons. Dev Neurobiol 2007; 67: 1687–1698.
Higuero AM, Sanchez-Ruiloba L, Doglio LE, Portillo F, Abad-Rodriguez J, Dotti CG et al. Kidins220/ARMS modulates the activity of microtubule-regulating proteins and controls neuronal polarity and development. J Biol Chem 2010; 285: 1343–1357.
Wu SH, Arevalo JC, Sarti F, Tessarollo L, Gan WB, Chao MV . Ankyrin repeat-rich membrane spanning/Kidins220 protein regulates dendritic branching and spine stability in vivo. Dev Neurobiol 2009; 69: 547–557.
Cesca F, Yabe A, Spencer-Dene B, Arrigoni A, Al-Qatari M, Henderson D et al. Kidins220/ARMS is an essential modulator of cardiovascular and nervous system development. Cell Death Dis 2011; e-pub ahead of print 3 November 2011, doi:10.1038/cddis.2011.108.
Lallemand Y, Luria V, Haffner-Krausz R, Lonai P . Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res 1998; 7: 105–112.
Bracale A, Cesca F, Neubrand VE, Newsome TP, Way M, Schiavo G . Kidins220/ARMS is transported by a kinesin-1-based mechanism likely to be involved in neuronal differentiation. Mol Biol Cell 2007; 18: 142–152.
Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo V, Baker D et al. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J 2006; 20: 1003–1005.
Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 2003; 5: 45–57.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L . VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 2006; 7: 359–371.
Levine ES, Dreyfus CF, Black IB, Plummer MR . Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA 1995; 92: 8074–8077.
Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N . Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci 1998; 18: 10231–10240.
Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen ZY, Lee FS et al. Long-distance control of synapse assembly by target-derived NGF. Neuron 2010; 67: 422–434.
Gale NW, Yancopoulos GD . Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 1999; 13: 1055–1066.
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M . Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998; 92: 735–745.
Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 1997; 19: 995–1005.
Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A . Postsynaptic induction of BDNF-mediated long-term potentiation. Science 2002; 295: 1729–1734.
Compagni A, Logan M, Klein R, Adams RH . Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell 2003; 5: 217–230.
Robinson EJ . Teratocarcinomas and Embryonic Stem Cells: A Practical Approach (Practical Approach Series). Oxford University Press: USA, 1987.
Bekkers JM, Stevens CF . Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 1991; 88: 7834–7838.
Pinheiro JC, Bates DM . Mixed Effects Models in S and S-Plus. Springer, 2002.
Team RDC. R: A language and environment for statistical computing ISBN 3-900051-07-0 2010.
Acknowledgements
We thank J Storm-Mathisen for help with the anatomical analysis, F Succol and M Nanni for technical assistance with primary neuronal cultures, C Ruhrberg for help with the analysis of Nrp1−/−-like phenotypes, G Kelly for statistical analysis, A Weston and T Arnett for microCT, N Corps for the CTAn software and scanning, CL Thomas for critical reading of the manuscript and members of the Molecular Neuropathobiology laboratory for helpful discussion. This study was supported by research grants from: Cancer Research UK (FC, AY, BS-D and GS); the Italian Institute of Technology (FC, JS-S, PB and FB); the Italian Ministry of University and Research (FB and PB); the Italian Ministry of Health Progetto Giovani (PB); the Compagnia di San Paolo, Torino (FB, PB); Telethon-Italy (Grant GGP09134 to FB and GGP09066 to PB); BBSRC (MAQ and MK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Companion paper of: doi:10.1038/cddis.2011.108
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Cesca, F., Yabe, A., Spencer-Dene, B. et al. Kidins220/ARMS mediates the integration of the neurotrophin and VEGF pathways in the vascular and nervous systems. Cell Death Differ 19, 194–208 (2012). https://doi.org/10.1038/cdd.2011.141
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.141
Keywords
This article is cited by
-
Kidins220 sets the threshold for survival of neural stem cells and progenitors to sustain adult neurogenesis
Cell Death & Disease (2023)
-
Kidins220 deficiency causes ventriculomegaly via SNX27-retromer-dependent AQP4 degradation
Molecular Psychiatry (2021)
-
Kidins220/ARMS controls astrocyte calcium signaling and neuron–astrocyte communication
Cell Death & Differentiation (2020)
-
Delivery of Brain-Derived Neurotrophic Factor by 3D Biocompatible Polymeric Scaffolds for Neural Tissue Engineering and Neuronal Regeneration
Molecular Neurobiology (2018)
-
Investigating the role of ankyrin-rich membrane spanning protein in human immunodeficiency virus type-1 Tat-induced microglia activation
Journal of NeuroVirology (2015)