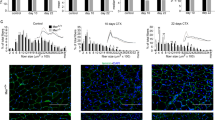
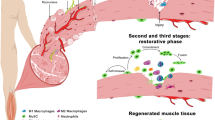
Abstract
Improving stem cell therapy is a major goal for the treatment of muscle diseases, where physiological muscle regeneration is progressively exhausted. Vessel-associated stem cells, such as mesoangioblasts (MABs), appear to be the most promising cell type for the cell therapy for muscular dystrophies and have been shown to significantly contribute to restoration of muscle structure and function in different muscular dystrophy models. Here, we report that melanoma antigen-encoding gene (MAGE) protein necdin enhances muscle differentiation and regeneration by MABs. When necdin is constitutively overexpressed, it accelerates their differentiation and fusion in vitro and it increases their efficacy in reconstituting regenerating myofibres in the α-sarcoglycan dystrophic mouse. Moreover, necdin enhances survival when MABs are exposed to cytotoxic stimuli that mimic the inflammatory dystrophic environment. Taken together, these data demonstrate that overexpression of necdin may be a crucial tool to boost therapeutic applications of MABs in dystrophic muscle.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Ab:
-
antibody
- αSMA:
-
alpha-smooth muscle actin
- α-SG:
-
alpha-sarcoglycan
- DMD:
-
Duchenne muscular dystrophy
- GFP:
-
green fluorescent protein
- MABs:
-
mesoangioblasts
- PDGFR:
-
platelet-derived growth factor receptor
- TA:
-
tibialis anterior
- TGFβ:
-
transforming growth factor beta
- TNFα:
-
tumor necrosis factor alpha
References
Zammit PS, Partridge TA, Yablonka-Reuveni Z . The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 2006; 54: 1177–1191.
Conboy IM, Rando TA . Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 2005; 4: 407–410.
Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 2002; 129: 2773–2783.
Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006; 444: 574–579.
Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 2003; 301: 487–492.
Barker PA, Salehi A . The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 2002; 67: 705–712.
Cassidy SB . Prader-Willi syndrome. J Med Genet 1997; 34: 917–923.
Gerard M, Hernandez L, Wevrick R, Stewart CL . Disruption of the mouse necdin gene results in early post-natal lethality. Nat Genet 1999; 23: 199–202.
Muscatelli F, Abrous DN, Massacrier A, Boccaccio I, Le Moal M, Cau P et al. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum Mol Genet 2000; 9: 3101–3110.
Deponti D, Francois S, Baesso S, Sciorati C, Innocenzi A, Broccoli V et al. Necdin mediates skeletal muscle regeneration by promoting myoblast survival and differentiation. J Cell Biol 2007; 179: 305–319.
Kuwajima T, Taniura H, Nishimura I, Yoshikawa K . Necdin interacts with the Msx2 homeodomain protein via MAGE-D1 to promote myogenic differentiation of C2C12 cells. J Biol Chem 2004; 279: 40484–40493.
Sciorati C, Touvier T, Buono R, Pessina P, Francois S, Perrotta C et al. Necdin is expressed in cachectic skeletal muscle to protect fibers from tumor-induced wasting. J Cell Sci 2009; 122 (Part 8): 1119–1125.
Pessina P, Conti V, Pacelli F, Rosa F, Doglietto GB, Brunelli S et al. Skeletal muscle of gastric cancer patients expresses genes involved in muscle regeneration. Oncol Rep 2010; 24: 741–745.
Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M . Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol 2007; Chapter 2: Unit 2B.1.
Brunelli S, Tagliafico E, De Angelis FG, Tonlorenzi R, Baesso S, Ferrari S et al. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ Res 2004; 94: 1571–1578.
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 2007; 9: 255–267.
Brunelli S, Rovere-Querini P . The immune system and the repair of skeletal muscle. Pharmacol Res 2008; 58: 117–121.
Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther 2007; 15: 867–877.
Cossu G, Biressi S . Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin Cell Dev Biol 2005; 16: 623–631.
Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G . PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 2008; 14: 973–978.
Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci USA 2007; 104: 264–269.
Donati C, Cencetti F, De Palma C, Rapizzi E, Brunelli S, Cossu G et al. TGFbeta protects mesoangioblasts from apoptosis via sphingosine kinase-1 regulation. Cell Signal 2009; 21: 228–236.
Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol 2007; 179: 33–40.
Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G et al. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci 2006; 119 (Part 24): 5114–5123.
Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 2006; 174: 231–243.
Zhu N-L, Wang J, Tsukamoto H . The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells. J Biol Chem 2010; 285: 30463–30471.
Tseng Y-H, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol 2005; 7: 601–611.
Uezumi A, Fukada S-i, Yamamoto N, Takeda Sai, Tsuchida K . Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010; 12: 143–152.
Biressi S, Rando TA . Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol 2010; 21: 845–854.
Hoshiya H, Kazuki Y, Abe S, Takiguchi M, Kajitani N, Watanabe Y et al. A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mol Ther 2009; 17: 309–317.
Weber J . Immunotherapy for melanoma. Curr Opin Oncol 2011; 23: 163–169.
Chapman EJ, Knowles MA . Necdin: a multi functional protein with potential tumor suppressor role? Mol Carcinog 2009; 48: 975–981.
Tagliafico E, Brunelli S, Bergamaschi A, De Angelis L, Scardigli R, Galli D et al. TGF{beta}/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J Cell Sci 2004; 117 (Part 19): 4377–4388.
Acknowledgements
This work has benefited from research funding from Telethon (GGP07013), the Italian Ministry of University and Research (PRIN 2009), the European Community's Seventh Framework Programme in the project ENDOSTEM (Grant agreement number 241440) to SB, and OPTISTEM (Grant agreement number 223098) to GC. We are also grateful to Jordi Diaz-Manera for technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by R De Maria
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Pessina, P., Conti, V., Tonlorenzi, R. et al. Necdin enhances muscle reconstitution of dystrophic muscle by vessel-associated progenitors, by promoting cell survival and myogenic differentiation. Cell Death Differ 19, 827–838 (2012). https://doi.org/10.1038/cdd.2011.160
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.160
Keywords
This article is cited by
-
The alpha subunit of Go modulates cell proliferation and differentiation through interactions with Necdin
Cell Communication and Signaling (2014)