Abstract
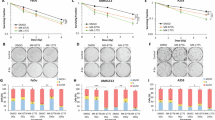
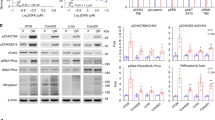
Reactivation of the p53 tumor-suppressor protein by small molecules like Nutlin-3 and RITA (reactivation of p53 and induction of tumor cell apoptosis) is a promising strategy for cancer therapy. The molecular mechanisms involved in the responses to RITA remain enigmatic. Several groups reported the induction of a p53-dependent DNA damage response. Furthermore, the existence of a p53-dependent S-phase checkpoint has been suggested, involving the checkpoint kinase Chk1. We have recently shown synergistic induction of apoptosis by RITA in combination with Nutlin-3, and we observed concomitant Chk2 phosphorylation. Therefore, we investigated whether Chk2 contributes to the cellular responses to RITA. Strikingly, the induction of apoptosis seemed entirely Chk2 dependent. Transcriptional activity of p53 in response to RITA required the presence of Chk2. A partial rescue of apoptosis observed in Noxa knockdown cells emphasized the relevance of p53 transcriptional activity for RITA-induced apoptosis. In addition, we observed an early p53- and Chk2-dependent block of DNA replication upon RITA treatment. Replicating cells seemed more prone to entering RITA-induced apoptosis. Furthermore, the RITA-induced DNA damage response, which was not a secondary effect of apoptosis induction, was strongly attenuated in cells lacking p53 or Chk2. In conclusion, we identified Chk2 as an essential mediator of the cellular responses to RITA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- RITA:
-
reactivation of p53 and induction of tumor cell apoptosis
- Wip1:
-
wild-type p53-induced phosphatase 1
- ATM:
-
ataxia telangiectasia mutated
- ATR:
-
ataxia telangiectasia and Rad3-related
- DNA-PK:
-
DNA-dependent protein kinase
- KAP1:
-
KRAB-interacting protein 1
- PUMA:
-
p53-upregulated modulator of apoptosis
- Hdm2:
-
human double minute 2
- Hdmx:
-
human double minute x
- PARP:
-
poly [ADP-ribose] polymerase
- shRNA:
-
short-hairpin RNA
- mRNA:
-
messenger RNA
- CI:
-
combination index
- EdU:
-
5-ethynyl-2′-deoxyuridine
References
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000; 408: 307–310.
Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 2004; 10: 1321–1328.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844–848.
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S et al. Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 2006; 49: 3432–3435.
Enge M, Bao W, Hedstrom E, Jackson SP, Moumen A, Selivanova G . MDM2-dependent downregulation of p21 and hnRNP K provides a switch between apoptosis and growth arrest induced by pharmacologically activated p53. Cancer Cell 2009; 15: 171–183.
Krajewski M, Ozdowy P, D’Silva L, Rothweiler U, Holak TA . NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nat Med 2005; 11: 1135–1136.
Rinaldo C, Prodosmo A, Siepi F, Moncada A, Sacchi A, Selivanova G et al. HIPK2 regulation by MDM2 determines tumor cell response to the p53-reactivating drugs nutlin-3 and RITA. Cancer Res 2009; 69: 6241–6428.
Hedstrom E, Eriksson S, Zawacka-Pankau J, Arner ES, Selivanova G . p53-dependent inhibition of TrxR1 contributes to the tumor-specific induction of apoptosis by RITA. Cell Cycle 2009; 8: 3576–3583.
Spinnler C, Hedstrom E, Li H, De Lange J, Nikulenkov F, Teunisse AF et al. Abrogation of Wip1 expression by RITA-activated p53 potentiates apoptosis induction via activation of ATM and inhibition of HdmX. Cell Death Differ 2011; 18: 1736–1745.
Yang J, Ahmed A, Poon E, Perusinghe N, de Haven BA, Box G et al. Small-molecule activation of p53 blocks hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia. Mol Cell Biol 2009; 29: 2243–2253.
Ahmed A, Yang J, Maya-Mendoza A, Jackson DA, Ashcroft M . Pharmacological activation of a novel p53-dependent S-phase checkpoint involving CHK-1. Cell Death Dis 2011; 2: e160.
de Lange J, Ly LV, Lodder K, Verlaan-de Vries M, Teunisse AF, Jager MJ et al. Synergistic growth inhibition based on small-molecule p53 activation as treatment for intraocular melanoma. Oncogene 2011; e-pub ahead of print 18 July 2011; doi:10.1038/onc.2011.309.
Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 2000; 14: 1448–1459.
Zhao H, Piwnica-Worms H . ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 2001; 21: 4129–4139.
Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE . Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 2000; 60: 5934–5936.
Buscemi G, Carlessi L, Zannini L, Lisanti S, Fontanella E, Canevari S et al. DNA damage-induced cell cycle regulation and function of novel Chk2 phosphoresidues. Mol Cell Biol 2006; 26: 7832–7845.
Meek DW . Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 2009; 9: 714–723.
Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de FM, Castano E et al. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood 2006; 107: 4109–4114.
Lam S, Lodder K, Teunisse AF, Rabelink MJ, Schutte M, Jochemsen AG . Role of Mdm4 in drug sensitivity of breast cancer cells. Oncogene 2010; 29: 2415–2426.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006; 444: 61–66.
Saha MN, Jiang H, Mukai A, Chang H . RITA inhibits multiple myeloma cell growth through induction of p53-mediated caspase-dependent apoptosis and synergistically enhances nutlin-induced cytotoxic responses. Mol Cancer Ther 2010; 9: 3041–3051.
Chou TC . Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58: 621–681.
Jack MT, Woo RA, Motoyama N, Takai H, Lee PW . DNA-dependent protein kinase and checkpoint kinase 2 synergistically activate a latent population of p53 upon DNA damage. J Biol Chem 2004; 279: 15269–15273.
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 1999; 18: 6845–6854.
Saito S, Goodarzi AA, Higashimoto Y, Noda Y, Lees-Miller SP, Appella E et al. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J Biol Chem 2002; 277: 12491–12494.
Blasina A, de IV W, Laus MC, Luyten WH, Parker AE, McGowan CH . A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol 1999; 9: 1–10.
Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J . The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 2001; 410: 842–847.
Lee JS, Collins KM, Brown AL, Lee CH, Chung JH . hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 2000; 404: 201–204.
Stevens C, Smith L, La Thangue NB . Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 2003; 5: 401–409.
Yang S, Kuo C, Bisi JE, Kim MK . PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat Cell Biol 2002; 4: 865–870.
Antoni L, Sodha N, Collins I, Garrett MD . CHK2 kinase: cancer susceptibility and cancer therapy –two sides of the same coin? Nat Rev Cancer 2007; 7: 925–936.
Ma CX, Janetka JW, Piwnica-Worms H . Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med 2011; 17: 88–96.
Chen CR, Wang W, Rogoff HA, Li X, Mang W, Li CJ . Dual induction of apoptosis and senescence in cancer cells by Chk2 activation: checkpoint activation as a strategy against cancer. Cancer Res 2005; 65: 6017–6021.
Carlessi L, Buscemi G, Larson G, Hong Z, Wu JZ, Delia D . Biochemical and cellular characterization of VRX0466617, a novel and selective inhibitor for the checkpoint kinase Chk2. Mol Cancer Ther 2007; 6: 935–944.
Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J 2002; 21: 5195–5205.
Flatten K, Dai NT, Vroman BT, Loegering D, Erlichman C, Karnitz LM et al. The role of checkpoint kinase 1 in sensitivity to topoisomerase I poisons. J Biol Chem 2005; 280: 14349–14355.
Macurek L, Lindqvist A, Voets O, Kool J, Vos HR, Medema RH . Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene 2010; 29: 2281–2291.
CompuSyn software for drug combinations and for general dose-effect analysis, and user's guide [computer program] 2007. ComboSyn Inc.: Paramus, NJ.
Acknowledgements
We thank Professor BR Ksander for providing the Mel202 cell line, Dr. B Vogelstein for providing the HCT116 cell lines, Dr. R Medema for the MCF-7/pRS, MCF-7/pRS-shWip1 cells and the Wip1-inducible U2OS cells U2TR, as well as Dr. G Selivanova for providing RITA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Oren
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
de Lange, J., Verlaan-de Vries, M., Teunisse, A. et al. Chk2 mediates RITA-induced apoptosis. Cell Death Differ 19, 980–989 (2012). https://doi.org/10.1038/cdd.2011.182
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.182
Keywords
This article is cited by
-
A quantitative LumiFluo assay to test inhibitory compounds blocking p53 degradation induced by human papillomavirus oncoprotein E6 in living cells
Scientific Reports (2018)
-
CRISPR-Cas9–based target validation for p53-reactivating model compounds
Nature Chemical Biology (2016)
-
Integrin α5β1 and p53 convergent pathways in the control of anti-apoptotic proteins PEA-15 and survivin in high-grade glioma
Cell Death & Differentiation (2016)
-
RITA (Reactivating p53 and Inducing Tumor Apoptosis) is efficient against TP53 abnormal myeloma cells independently of the p53 pathway
BMC Cancer (2014)
-
Cytoplasmic parafibromin/hCdc73 targets and destabilizes p53 mRNA to control p53-mediated apoptosis
Nature Communications (2014)