Abstract
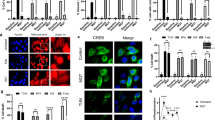
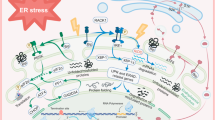
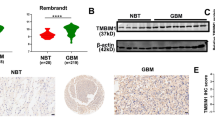
Transmembrane BAX inhibitor motif-containing (TMBIM)-6, also known as BAX-inhibitor 1 (BI-1), is an anti-apoptotic protein that belongs to a putative family of highly conserved and poorly characterized genes. Here we report the function of TMBIM3/GRINA in the control of cell death by endoplasmic reticulum (ER) stress. Tmbim3 mRNA levels are strongly upregulated in cellular and animal models of ER stress, controlled by the PERK signaling branch of the unfolded protein response. TMBIM3/GRINA synergies with TMBIM6/BI-1 in the modulation of ER calcium homeostasis and apoptosis, associated with physical interactions with inositol trisphosphate receptors. Loss-of-function studies in D. melanogaster demonstrated that TMBIM3/GRINA and TMBIM6/BI-1 have synergistic activities against ER stress in vivo. Similarly, manipulation of TMBIM3/GRINA levels in zebrafish embryos revealed an essential role in the control of apoptosis during neuronal development and in experimental models of ER stress. These findings suggest the existence of a conserved group of functionally related cell death regulators across species beyond the BCL-2 family of proteins operating at the ER membrane.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 2-APB:
-
2-aminoethoxydiphenyl borate
- ATF4:
-
activating transcription factor 4
- ATF6:
-
activating transcription factor 6
- BI-1:
-
bax inhibitor 1 protein
- ERAD:
-
endoplasmic reticulum-associated degradation
- Est:
-
estaurosporine
- Eto:
-
etoposide
- GAAP:
-
golgi anti-apoptotic proteín
- GFP:
-
green fluorescent protein
- GHITM:
-
growth-hormone inducible transmembrane protein
- GRINA:
-
glutamate receptor, ionotropic, N-methyl D-asparate-associated protein 1
- HA:
-
human influenza hemagglutinin
- hpf:
-
hours post fertilization
- IP3:
-
inositol 1,4,5-trisphosphate
- IP3-R:
-
inositol 1,4,5-triphosphate receptor
- Mo:
-
morpholine
- shRNA:
-
small hairpin RNA
- Thg:
-
thapsigargin
- Tm:
-
tunicamycin
- TMBIM:
-
transmembrane BAX inhibitor motif-containing
- UPR:
-
unfolded protein response
References
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Hengartner MO . Programmed cell death in invertebrates. Curr Opin Genet Dev 1996; 6: 34–38.
Sevrioukov EA, Burr J, Huang EW, Assi HH, Monserrate JP, Purves DC et al. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis 2007; 45: 184–193.
Galindo KA, Lu WJ, Park JH, Abrams JM . The Bax/Bak ortholog in Drosophila, Debcl, exerts limited control over programmed cell death. Development 2009; 136: 275–283.
Robinson KS, Clements A, Williams AC, Berger CN, Frankel G . Bax inhibitor 1 in apoptosis and disease. Oncogene 2011; 30: 2391–2400.
Reimers K, Choi CY, Bucan V, Vogt PM . The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr Mol Med 2008; 8: 148–156.
Hu L, Smith TF, Goldberger G . LFG: a candidate apoptosis regulatory gene family. Apoptosis 2009; 14: 1255–1265.
Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell 2004; 15: 355–366.
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell 2009; 33: 679–691.
Xu Q, Reed JC . Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell 1998; 1: 337–346.
Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J 2011; 30: 4465–4478.
Hetz C, Martinon F, Rodriguez D, Glimcher LH . The unfolded protein response: integrating stress signals through the stress sensor IRE1{alpha}. Physiol Rev 2011; 91: 1219–1243.
Hetz C, Glimcher LH . Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol Cell 2009; 35: 551–561.
Woehlbier U, Hetz C . Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci 2011; 36: 329–337.
Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K . The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem 2011; 149: 507–518.
Gubser C, Bergamaschi D, Hollinshead M, Lu X, van Kuppeveld FJ, Smith GL . A new inhibitor of apoptosis from vaccinia virus and eukaryotes. PLoS Pathog 2007; 3: e17.
Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol 2000; 148: 857–862.
Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci U S A 2009; 106: 14397–14402.
Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A 2005; 102: 105–110.
Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M et al. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron 2010; 68: 865–878.
Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 2009; 186: 783–792.
Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 2008; 27: 285–299.
Mori K . Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 2009; 146: 743–750.
Metzstein MM, Hengartner MO, Tsung N, Ellis RE, Horvitz HR . Transcriptional regulator of programmed cell death encoded by Caenorhabditis elegans gene ces-2. Nature 1996; 382: 545–547.
Huckelhoven R . BAX Inhibitor-1, an ancient cell death suppressor in animals and plants with prokaryotic relatives. Apoptosis 2004; 9: 299–307.
Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM . LFG: an anti-apoptotic gene that provides protection from Fas-mediated cell death. Proc Natl Acad Sci U S A 1999; 96: 12667–12672.
Zhao H, Ito A, Kimura SH, Yabuta N, Sakai N, Ikawa M et al. RECS1 deficiency in mice induces susceptibility to cystic medial degeneration. Genes Genet Syst 2006; 81: 41–50.
Zhao H, Ito A, Sakai N, Matsuzawa Y, Yamashita S, Nojima H . RECS1 is a negative regulator of matrix metalloproteinase-9 production and aged RECS1 knockout mice are prone to aortic dilation. Circ J 2006; 70: 615–624.
Shukla S, Fujita K, Xiao Q, Liao Z, Garfield S, Srinivasula SM . A shear stress responsive gene product PP1201 protects against Fas-mediated apoptosis by reducing Fas expression on the cell surface. Apoptosis 2011; 16: 162–173.
de Mattia F, Gubser C, van Dommelen MM, Visch HJ, Distelmaier F, Postigo A et al. Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell 2009; 20: 3638–3645.
Oka T, Sayano T, Tamai S, Yokota S, Kato H, Fujii G et al. Identification of a novel protein MICS1 that is involved in maintenance of mitochondrial morphology and apoptotic release of cytochrome c. Mol Biol Cell 2008; 19: 2597–2608.
Yamaji T, Nishikawa K, Hanada K . Transmembrane BAX inhibitor motif containing (TMBIM) family proteins perturbs a trans-Golgi network enzyme, Gb3 synthase, and reduces Gb3 biosynthesis. J Biol Chem 2010; 285: 35505–35518.
Kumar KN, Tilakaratne N, Johnson PS, Allen AE, Michaelis EK . Cloning of cDNA for the glutamate-binding subunit of an NMDA receptor complex. Nature 1991; 354: 70–73.
Hollmann M, Heinemann S . Cloned glutamate receptors. Annu Rev Neurosci 1994; 17: 31–108.
Bonaglia MC, Giorda R, Tenconi R, Pessina M, Pramparo T, Borgatti R et al. A 2.3 Mb duplication of chromosome 8q24.3 associated with severe mental retardation and epilepsy detected by standard karyotype. Eur J Hum Genet 2005; 13: 586–591.
Nielsen JA, Chambers MA, Romm E, Lee LY, Berndt JA, Hudson LD . Mouse transmembrane BAX inhibitor Motif 3 (Tmbim3) encodes a 38 kDa transmembrane protein expressed in the central nervous system. Mol Cell Biochem 2011; 357: 73–81.
Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 2009; 23: 2294–2306.
Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007; 448: 151–156.
Pinton P, Brini M, Bastianutto C, Tuft RA, Pozzan T, Rizzuto R . New light on mitochondrial calcium. Biofactors 1998; 8: 243–253.
Acknowledgements
We apologize to all colleagues whose work could not be cited owing to space limitations. We thank David Ron for providing IRE1α and PERK-deficient cells, Dr. Ann-Hwee Lee for providing and generating IRE1α KO-reconstituted cells, and Dr. NirHacohen and the Broad Institute (Boston, MA, USA) for providing shRNA lentiviral constructs. We also thank Dr. John Reed for kindly providing bi-1−/−cells and Dr. Randal Kaufman for providing ATF6α-deficient cells. We thank Peter Thielen for technical assistant in cloning. We thank Cecilia Zuñigafor for his technical support with fluorescence-activated cell sorting analysis, Tomas Luyten for technical support with 45Ca2+-flux assays and Jan Parys for helpful discussions. This work was supported by the FONDECYT no. 1100176, Alzheimer's Association, Muscular Dystrophy Association, Michael J Fox Foundation for Parkinson Research, North American Spine Society, and ICGEB (to CH); FONDECYT no.1090272 (JS), FONDECYT no. 11090324(AC), Howard Hughes Medical Institute (INTNL 55005940), FONDECYT (1090242) and the Millennium Initiative (ICM P07-048-F) (MC); FONDECYT no. 3100033 (DR); CONICYT Doctoral fellowship (DR-R, GM); UCH-0606 (DR-R); CONICYT no. 24090143 (DR-R), Millennium Nucleus no. P07-048-F (CH, MC, JS); FONDAP Grant no. 15010006 (RA, AS and CH); Grants STRT1/10/044 (GB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by H Ichijo
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Rojas-Rivera, D., Armisén, R., Colombo, A. et al. TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell Death Differ 19, 1013–1026 (2012). https://doi.org/10.1038/cdd.2011.189
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.189
Keywords
This article is cited by
-
miR-125a-3p regulates apoptosis by suppressing TMBIM4 in lens epithelial cells
International Ophthalmology (2022)
-
Genotoxic stress triggers the activation of IRE1α-dependent RNA decay to modulate the DNA damage response
Nature Communications (2020)
-
PRKCSH contributes to tumorigenesis by selective boosting of IRE1 signaling pathway
Nature Communications (2019)
-
Transmembrane protein GRINA modulates aerobic glycolysis and promotes tumor progression in gastric cancer
Journal of Experimental & Clinical Cancer Research (2018)
-
BCL-2 family: integrating stress responses at the ER to control cell demise
Cell Death & Differentiation (2017)