Abstract
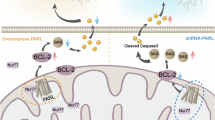
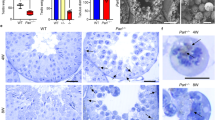
The mitochondrial rhomboid protease Parl governs apoptosis, morphology, metabolism and might be implicated in Parkinson's disease, but the structural basis of its activity and complex regulation remain unknown. We report the discovery of γ-cleavage, a proteolytic event on the loop connecting the first transmembrane helix (TMH) of Parl to the 6-TMH catalytic rhomboid domain of the protease. This cleavage disrupts the ‘1+6’ structure that defines every mitochondrial rhomboid and generates a new form of Parl, PROD (Parl-rhomboid-domain). Structure–function analysis of Parl suggests that γ-cleavage could be implicated in eliminating Parl proteolytic activity, and structural modeling of PROD reveals structural conservation with the bacterial rhomboid GlpG. However, unlike bacterial rhomboids, which employ a diad-based mechanism of catalysis, Parl appears to use a conserved mitochondrial rhomboid-specific Asp residue on TMH-5 in a triad-based mechanism of catalysis. This work provides unexpected insights into the structural determinants regulating Parl stability and activity in vivo, and reveals a complex cascade of proteolytic events controlling the function of the protease in the mitochondrion.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MAMP:
-
mature mitochondrial Parl protein
- PACT:
-
Parl C-terminal protein
- PROD:
-
Parl rhomboid domain protein
- TMH:
-
transmembrane helix
- IMM:
-
inner mitochondrial membrane
- DDM:
-
n-dodecyl-β-D-maltoside
References
Koonin EV, Makarova KS, Rogozin IB, Davidovic L, Letellier MC, Pellegrini L . The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers. Genome Biol 2003; 4: R19.
Urban S, Lee JR, Freeman M . Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 2001; 107: 173–182.
Urban S . Rhomboid proteins: conserved membrane proteases with divergent biological functions. Genes Dev 2006; 20: 3054–3068.
Urban S, Wolfe MS . Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proc Natl Acad Sci USA 2005; 102: 1883–1888.
Wang Y, Zhang Y, Ha Y . Crystal structure of a rhomboid family intramembrane protease. Nature 2006; 444: 179–180.
Wu Z, Yan N, Feng L, Oberstein A, Yan H, Baker RP et al. Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry. Nat Struct Mol Biol 2006; 13: 1084–1091.
Ben-Shem A, Fass D, Bibi E . Structural basis for intramembrane proteolysis by rhomboid serine proteases. Proc Natl Acad Sci USA 2007; 104: 462–466.
Lemieux MJ, Fischer SJ, Cherney MM, Bateman KS, James MN . The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis. Proc Natl Acad Sci USA 2007; 104: 750–754.
Bondar AN, del Val C, White SH . Rhomboid protease dynamics and lipid interactions. Structure 2009; 17: 395–405.
Clemmer KM, Sturgill GM, Veenstra A, Rather PN . Functional characterization of Escherichia coli GlpG and additional rhomboid proteins using an aarA mutant of Providencia stuartii. J Bacteriol 2006; 188: 3415–3419.
Erez E, Fass D, Bibi E . How intramembrane proteases bury hydrolytic reactions in the membrane. Nature 2009; 459: 371–378.
Ha Y . Structure and mechanism of intramembrane protease. Semin Cell Dev Biol 2009; 20: 240–250.
Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 2006; 126: 163–175.
Jeyaraju DV, Xu L, Letellier MC, Bandaru S, Zunino R, Berg EA et al. Phosphorylation and cleavage of presenilin-associated rhomboid-like protein (PARL) promotes changes in mitochondrial morphology. Proc Natl Acad Sci USA 2006; 103: 18562–18567.
Civitarese AE, MacLean PS, Carling S, Kerr-Bayles L, McMillan RP, Pierce A et al. Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL. Cell Metab 2010; 11: 412–426.
Sík A, Passer BJ, Koonin EV, Pellegrini L . Self-regulated cleavage of the mitochondrial intramembrane-cleaving protease PARL yields Pbeta, a nuclear-targeted peptide. J Biol Chem 2004; 279: 15323–15329.
Hill RB, Pellegrini L . The PARL family of mitochondrial rhomboid proteases. Semin Cell Dev Biol 2010; 21: 582–592.
Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh S et al. PINK1 Cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 2010; 20: 867–879.
Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ . Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 2010; 191: 933–942.
Shi G, Lee JR, Grimes DA, Racacho L, Ye D, Yang H et al. Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson's disease. Hum Mol Genet 2011 (E-pub ahead of print 25 February 2011).
Baker RP, Young K, Feng L, Shi Y, Urban S . Enzymatic analysis of a rhomboid intramembrane protease implicates transmembrane helix 5 as the lateral substrate gate. Proc Natl Acad Sci USA 2007; 104: 8257–8262.
Koonin EV, Wolf YI . Constraints and plasticity in genome and molecular-phenome evolution. Nat Rev Genetics 2010; 11: 487–498.
Rawlings ND, Barrett AJ . Families of serine peptidases. Methods Enzymol 1994; 244: 19–61.
MacKenzie KR, Fleming KG . Association energetics of membrane spanning alpha-helices. Curr Opin Struct Biol 2008; 18: 412–419.
Maegawa S, Koide K, Ito K, Akiyama Y . The intramembrane active site of GlpG, an E. coli rhomboid protease, is accessible to water and hydrolyses an extramembrane peptide bond of substrates. Mol Microbiol 2007; 64: 435–447.
Tsukada H, Blow DM . Structure of alpha-chymotrypsin refined at 1.68 A resolution. J Mol Biol 1985; 184: 703–711.
Bernstein HD, Poritz MA, Strub K, Hoben PJ, Brenner S, Walter P . Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature 1989; 340: 482–486.
O′Neil KT, Erickson-Viitanen S, DeGrado WF . Photolabeling of calmodulin with basic, amphiphilic alpha-helical peptides containing p-benzoylphenylalanine. J Biol Chem 1989; 264: 14571–14578.
Gellman SH . On the role of methionine residues in the sequence-independent recognition of nonpolar protein surfaces. Biochemistry 1991; 30: 6633–6636.
McGregor MJ, Islam SA, Sternberg MJ . Analysis of the relationship between side-chain conformation and secondary structure in globular proteins. J Mol Biol 1987; 198: 295–310.
Jeyaraju DV, Cisbani G, De Brito OM, Koonin EV, Pellegrini L . Hax1 lacks BH modules and is peripherally associated to heavy membranes: implications for Omi/HtrA2 and PARL activity in the regulation of mitochondrial stress and apoptosis. Cell Death Differ 2009; 16: 1622–1629.
Wang Y, Ha Y . Open-cap conformation of intramembrane protease GlpG. Proc Natl Acad Sci USA 2007; 104: 2098–2102.
Wang Y, Maegawa S, Akiyama Y, Ha Y . The role of L1 loop in the mechanism of rhomboid intramembrane protease GlpG. J Mol Biol 2007; 374: 1104–1113.
Muñoz V, Serrano L . Development of the multiple sequence approximation within the AGADIR model of alpha-helix formation: comparison with Zimm-Bragg and Lifson-Roig formalisms. Biopolymers 1997; 41: 495–509.
Arnold K, Bordoli L, Kopp J, Schwede T . The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22: 195–201.
Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY et al. Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci 2007 Chapter 2: Unit 2.9.
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 2010; 66: 12–21.
DeLano WL . The PyMOL molecular graphics system. DeLano Scientific 2002.
Acknowledgements
We thank Li Xu for assistance in mitochondria morphology analysis, Katalin Toth for support with the statistical analysis, and former members of the Pellegrini Laboratory for technical assistance. This study was supported by a NIH grant to RBH (RO1GM067180), by a CIHR grant to LP (MOP-82718), and by a FRSQ senior scholarship to LP. DVJ is a CIHR Canada Graduate Scholar.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by L Scorrano
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Jeyaraju, D., McBride, H., Hill, R. et al. Structural and mechanistic basis of Parl activity and regulation. Cell Death Differ 18, 1531–1539 (2011). https://doi.org/10.1038/cdd.2011.22
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.22
Keywords
This article is cited by
-
The rhomboid protease family: a decade of progress on function and mechanism
Genome Biology (2011)
-
Derlin-1 is a rhomboid pseudoprotease required for the dislocation of mutant α-1 antitrypsin from the endoplasmic reticulum
Nature Structural & Molecular Biology (2011)