Abstract
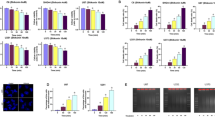
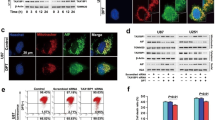
Apoptosis inducing factor (AIF) is a mitochondrial oxidoreductase that scavenges reactive oxygen species under normal conditions. Under certain stresses, such as exposure to N -methyl- N′ -nitro- N′ -nitrosoguanidine (MNNG), AIF is truncated and released from the mitochondria and translocated into the nucleus, where the truncated AIF (tAIF) induces caspase-independent cell death. However, it is unknown how cells decide to kill themselves or operate ways to survive when they encounter stresses that induce the release of tAIF. Here, we demonstrated that USP2 and CHIP contribute to the control of tAIF stability. USP2 deubiquitinated and stabilized tAIF, thus promoting AIF-mediated cell death. In contrast, CHIP ubiquitinated and destabilized tAIF, thus preventing the cell death. Consistently, CHIP-deficient cells showed an increased sensitivity to MNNG. On the other hand, knockdown of USP2 attenuated MNNG-induced cell death. Moreover, exposure to MNNG caused a dramatic decrease in CHIP level, but not that of USP2, concurrent with cell shrinkage and chromatin condensation. These findings indicate that CHIP and USP2 show antagonistic functions in the control of AIF-mediated cell death, and implicate the role of the enzymes as a switch for cells to live or die under stresses that cause tAIF release.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AIF:
-
apoptosis inducing factor
- CHIP:
-
C-terminus of Hsp70-interacting protein
- FITC:
-
fluorescein isothiocyanate
- GSH:
-
glutathione
- GST:
-
glutathione S-transferase
- MNNG:
-
N-methyl-N′-nitro-N′-nitrosoguanidine
- NEM:
-
N-ethylmaleimide
- NTA:
-
nitrilotriacetic acid
- PARP:
-
poly(ADP-ribose) polymerase-1
- PMSF:
-
phenylmethylsulfonyl fluoride
- TRITC:
-
tetramethylrhodamine isothiocyanate
- USP2:
-
ubiquitin-specific protease-2
References
Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N et al. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett 2000; 476: 118–123.
Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN et al. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 2002; 419: 367–374.
Brown D, Yu BD, Joza N, Benit P, Meneses J, Firpo M et al. Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc Natl Acad Sci USA 2006; 103: 9918–9923.
Joza N, Galindo K, Pospisilik JA, Benit P, Rangachari M, Kanitz EE et al. The molecular archaeology of a mitochondrial death effector: AIF in Drosophila. Cell Death Differ 2008; 15: 1009–1018.
Joza N, Oudit GY, Brown D, Benit P, Kassiri Z, Vahsen N et al. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 2005; 25: 10261–10272.
Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007; 131: 476–491.
Hangen E, Blomgren K, Benit P, Kroemer G, Modjtahedi N . Life with or without AIF. Trends Biochem Sci 2010; 35: 278–287.
Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA . AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle 2007; 6: 2612–2619.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397: 441–446.
Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002; 297: 259–263.
Wesierska-Gadek J, Gueorguieva M, Schloffer D, Uhl M, Wojciechowski J . Non-apoptogenic killing of hela cervical carcinoma cells after short exposure to the alkylating agent N-methyl-N’-nitro-N-nitrosoguanidine (MNNG). J Cell Biochem 2003; 89: 1222–1234.
Otera H, Ohsakaya S, Nagaura ZI, Ishihara N, Mihara K . Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J 2005; 24: 1375–1386.
Artus C, Boujrad H, Bouharrour A, Brunelle MN, Hoos S, Yuste VJ et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J 2010; 29: 1585–1599.
Yuste VJ, Moubarak RS, Delettre C, Bras M, Sancho P, Robert N et al. Cysteine protease inhibition prevents mitochondrial apoptosis-inducing factor (AIF) release. Cell Death Differ 2005; 12: 1445–1448.
Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J et al. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol 2007; 27: 4844–4862.
Wang Y, Kim NS, Li X, Greer PA, Koehler RC, Dawson VL et al. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos). J Neurochem 2009; 110: 687–696.
Hangen E, De Zio D, Bordi M, Zhu C, Dessen P, Caffin F et al. A brain-specific isoform of mitochondrial apoptosis-inducing factor: AIF2. Cell Death Differ 2010; 17: 1155–1166.
Hershko A, Ciechanover A . The ubiquitin system. Annu Rev Biochem 1998; 67: 425–479.
Hochstrasser M . Ubiquitin-dependent protein degradation. Annu Rev Genet 1996; 30: 405–439.
Kim JH, Park KC, Chung SS, Bang O, Chung CH . Deubiquitinating enzymes as cellular regulators. J Biochem 2003; 134: 9–18.
Wilkinson KD . Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 1997; 11: 1245–1256.
Murata S, Minami Y, Minami M, Chiba T, Tanaka K . CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep 2001; 2: 1133–1138.
Cyr DM, Höhfeld J, Patterson C . Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci 2002; 27: 368–375.
Kampinga HH, Kanon B, Salomons FA, Kabakov AE, Patterson C . Overexpression of the cochaperone CHIP enhances Hsp70-dependent folding activity in mammalian cells. Mol Cell Biol 2003; 23: 4948–4958.
Park KC, Kim JH, Choi EJ, Min SW, Rhee S, Baek SH et al. Antagonistic regulation of myogenesis by two deubiquitinating enzymes, UBP45 and UBP69. Proc Natl Acad Sci USA 2002; 99: 9733–9738.
Baek SH, Choi KS, Yoo YJ, Cho JM, Baker RT, Tanaka K et al. Molecular cloning of a novel ubiquitin-specific protease, UBP41, with isopeptidase activity in chick skeletal muscle. J Biol Chem 1997; 272: 25560–25565.
Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK . The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J 2007; 26: 976–986.
Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 2004; 5: 253–261.
Gewies A, Grimm S . UBP41 is a proapoptotic ubiquitin-specific protease. Cancer Res 2003; 63: 682–688.
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3: 839–843.
Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J et al. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem 2001; 276: 42938–42944.
Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem 2005; 94: 1254–1263.
Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C et al. Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell 2005; 20: 525–538.
Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C . CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 2006; 440: 551–555.
Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ . The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem 2005; 280: 23727–23734.
Lorenzo HK, Susin SA . Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist Updat 2007; 10: 235–255.
Rugo H, Shtivelman E, Perez A, Vogel C, Franco S, Tan Chiu E et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res Treat 2007; 105: 17–28.
Hong SC, Goto Y, Lanzino G, Soleau S, Kassell NF, Lee KS . Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke 1994; 25: 663–669.
Natsume T, Yamauchi Y, Nakayama H, Shinkawa T, Yanagida M, Takahashi N et al. A direct nanoflow liquid chromatography—tandem mass spectrometry system for interaction proteomics. Anal Chem 2002; 74: 4725–4733.
Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S . Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptois. J Biol Chem 2002; 277: 29803–29809.
Acknowledgements
We thank Dr. G Kroemer for providing pcDNA3-AIF. We also thank Ms. SH Ka for her excellent technical assistance. This work was supported by grants from KRF (KRF-2005-084-C00025) and KOSEF (M10533010001-05N3301). KH Oh was the recipient of TJ Park postdoctoral fellowship. SW Yang and JM Park were the recipients of BK21 fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Vandenabeele
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Oh, K., Yang, S., Park, J. et al. Control of AIF-mediated cell death by antagonistic functions of CHIP ubiquitin E3 ligase and USP2 deubiquitinating enzyme. Cell Death Differ 18, 1326–1336 (2011). https://doi.org/10.1038/cdd.2011.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.3
Keywords
This article is cited by
-
Ubiquitin ligase CHIP regulates OTUD3 stability and suppresses tumour metastasis in lung cancer
Cell Death & Differentiation (2020)
-
Observation of Parthanatos Involvement in Diminished Ovarian Reserve Patients and Melatonin’s Protective Function Through Inhibiting ADP-Ribose (PAR) Expression and Preventing AIF Translocation into the Nucleus
Reproductive Sciences (2020)
-
Multifaceted C-terminus of HSP70-interacting protein regulates tumorigenesis via protein quality control
Archives of Pharmacal Research (2019)
-
A novel function of cIAP1 as a mediator of CHIP-driven eIF4E regulation
Scientific Reports (2017)
-
Phosphorylation of CHIP at Ser20 by Cdk5 promotes tAIF-mediated neuronal death
Cell Death & Differentiation (2016)