Abstract
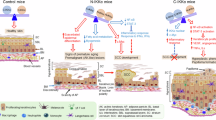
Inhibitor of nuclear factor κB kinase-α (IKKα) is required for maintaining skin homeostasis and preventing skin tumorigenesis. However, its signaling has not been extensively investigated. In the present study, we generated two mouse lines that expressed different levels of transgenic IKKα in the basal epidermis under the control of keratin-5 promoter and further evaluated their effects on the major pathways of inflammation, proliferation, and differentiation in the skin. Regardless of the transgenic IKKα levels, the mice develop normally. Because IKKα deletion in keratinocytes blocks terminal differentiation and induces epidermal hyperplasia and skin inflammation, we depleted the endogenous IKKα in these transgenic mice and found that the transgenic IKKα represses epidermal thickness and induces terminal differentiation in a dose-dependent manner. Also, transgenic IKKα was found to elevate expression of Max dimer protein 1 (Mad1) and ovo-like 1, c-Myc antagonists, but repress activities of epidermal growth factor receptor (EGFR), extracellular signal-regulated kinase (ERK), Jun-amino-terminal kinases, c-Jun, signal transducer and activator of transcription 3 (Stat3), and growth factor levels in a dose-dependent fashion in the skin. Moreover, EGFR reduction represses IKKα deletion-induced excessive ERK, Stat3 and c-Jun activities, and skin inflammation. These new findings indicate that elevated IKKα expression not only represses epidermal thickness and induces terminal differentiation, but also suppresses skin inflammation by an integrated loop. Thus, IKKα maintains skin homeostasis through a broad range of signaling pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- IKKα:
-
inhibitor of nuclear factor κB kinase-α
- Mad1:
-
Max dimer protein 1
- EGFR:
-
epidermal growth factor receptor
- Stat3:
-
signal transducer and activator of transcription 3
- HB-EGF:
-
heparin-binding epidermal growth factor
- NF-κB:
-
nuclear factor κB
- IκB:
-
inhibitor of NF-κB
References
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M . A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 1997; 388: 548–554.
Ghosh S, Karin M . Missing pieces in the NF-kappaB puzzle. Cell 2002; 109 (Suppl): S81–S96.
Baud V, Karin M . Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 2009; 8: 33–40.
Zhu F, Park E, Liu B, Xia X, Fischer SM, Hu Y . Critical role of IkB kinase alpha in embryonic development and skin carcinogenesis. Histol Histopathol 2009; 24: 265–271.
Orlowski RZ, Baldwin Jr AS . NF-kappaB as a therapeutic target in cancer. Trends Mol Med 2002; 8: 385–389.
Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K et al. Crosstalk between keratinocytes and adaptive immune cells in an IkappaBalpha protein-mediated inflammatory disease of the skin. Immunity 2007; 27: 296–307.
Beg AA, Sha WC, Bronson RT, Baltimore D . Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev 1995; 9: 2736–2746.
Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 1999; 284: 316–320.
Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T et al. Limb and skin abnormalities in mice lacking IKKα. Science 1999; 284: 313–316.
Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC et al. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev 1999; 13: 1322–1328.
Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M et al. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med 1999; 189: 1839–1845.
Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 2000; 5: 969–979.
Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKgamma-deficient mice. Genes Dev 2000; 14: 854–862.
Sil AK, Maeda S, Sano Y, Roop DR, Karin M . IKKα acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature 2004; 428: 660–664.
Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K et al. IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell 2008; 14: 212–225.
Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J et al. A critical role for IkappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc Natl Acad Sci USA 2006; 103: 17202–17207.
Maeda G, Chiba T, Kawashiri S, Satoh T, Imai K . Epigenetic inactivation of IkappaB kinase-alpha in oral carcinomas and tumor progression. Clin Cancer Res 2007; 13: 5041–5047.
Van Waes C, Yu M, Nottingham L, Karin M . Inhibitor-kappaB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clin Cancer Res 2007; 13: 4956–4959.
Marinari B, Moretti F, Botti E, Giustizieri ML, Descargues P, Giunta A et al. The tumor suppressor activity of IKKalpha in stratified epithelia is exerted in part via the TGF-beta antiproliferative pathway. Proc Natl Acad Sci USA 2008; 105: 17091–17096.
Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P et al. IKKalpha is a critical coregulator of a Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA 2008; 105: 2487–2492.
Li N, Karin M . Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA 1998; 95: 13012–13017.
Saleem M, Afaq F, Adhami VM, Mukhtar H . Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004; 23: 5203–5214.
Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 2007; 129: 903–914.
Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T et al. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res 2007; 67: 9158–9168.
Page A, Navarro M, Garin M, Perez P, Casanova ML, Moreno R et al. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J Invest Dermatol 2010; 130: 1598–1610.
Yoshida K, Hu Y, Karin M . IkappaB kinase alpha is essential for development of the mammalian cornea and conjunctiva. Invest Ophthalmol Vis Sci 2000; 41: 3665–3669.
Xia X, Park E, Liu B, Willette-Brown J, Gong W, Wang J et al. Reduction of IKKalpha expression promotes chronic ultraviolet B exposure-induced skin inflammation and carcinogenesis. Am J Pathol 2010; 176: 2500–2508.
Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M . IKKα controls formation of the epidermis independently of NF-κB. Nature 2001; 410: 710–714.
Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M . The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 1997; 91: 243–252.
Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 1997; 278: 860–866.
Seitz CS, Lin Q, Deng H, Khavari PA . Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc Natl Acad Sci USA 1998; 95: 2307–2312.
Lind MH, Rozell B, Wallin RP, van Hogerlinden M, Ljunggren HG, Toftgard R et al. Tumor necrosis factor receptor 1-mediated signaling is required for skin cancer development induced by NF-kappaB inhibition. Proc Natl Acad Sci USA 2004; 101: 4972–4977.
Zenz R, Scheuch H, Martin P, Frank C, Eferl R, Kenner L et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell 2003; 4: 879–889.
Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther 2008; 10: 201–211.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Sano S, Chan KS, DiGiovanni J . Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J Dermatol Sci 2008; 50: 1–14.
Chan KS, Sano S, Kataoka K, Abel E, Carbajal S, Beltran L et al. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene 2008; 27: 1087–1094.
Kim DJ, Angel JM, Sano S, DiGiovanni J . Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene 2009; 28: 950–960.
Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J 1999; 18: 4657–4668.
Acknowledgements
This work was supported by National Cancer Institute (NCI) grants CA102510, CA117314 (to YH), and CA105345 (to SMF), and by NCI intramural funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Karin
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, B., Willette-Brown, J., Liu, S. et al. IKKα represses a network of inflammation and proliferation pathways and elevates c-Myc antagonists and differentiation in a dose-dependent manner in the skin. Cell Death Differ 18, 1854–1864 (2011). https://doi.org/10.1038/cdd.2011.56
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2011.56
Keywords
This article is cited by
-
A TNFR1–UBCH10 axis drives lung squamous cell carcinoma dedifferentiation and metastasis through a cell-autonomous signaling loop
Cell Death & Disease (2022)
-
miR-24 affects hair follicle morphogenesis targeting Tcf-3
Cell Death & Disease (2013)