Abstract
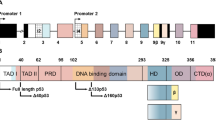
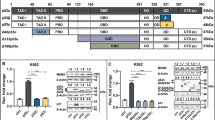
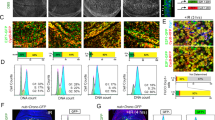
Irradiated or injured cells enter apoptosis, and in turn, promote proliferation of surrounding unaffected cells. In Drosophila, apoptotic cells have an active role in proliferation, where the caspase Dronc and p53 induce mitogen expression and growth in the surrounding tissues. The Drosophila p53 gene structure is conserved and encodes at least two protein isoforms: a full-length isoform (Dp53) and an N-terminally truncated isoform (DΔNp53). Historically, DΔNp53 was the first p53 isoform identified and was thought to be responsible for all p53 biological activities. It was shown that DΔNp53 induces apoptosis by inducing the expression of IAP antagonists, such as Reaper. Here we investigated the roles of Dp53 and DΔNp53 in apoptosis and apoptosis-induced proliferation. We found that both isoforms were capable of activating apoptosis, but that they each induced distinct IAP antagonists. Expression of DΔNp53 induced Wingless (Wg) expression and enhanced proliferation in both ‘undead cells’ and in ‘genuine’ apoptotic cells. In contrast to DΔNp53, Dp53 did not induce Wg expression in the absence of the endogenous p53 gene. Thus, we propose that DΔNp53 is the main isoform that regulates apoptosis-induced proliferation. Understanding the roles of Drosophila p53 isoforms in apoptosis and in apoptosis-induced proliferation may shed new light on the roles of p53 isoforms in humans, with important implications in cancer biology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bergmann A, Steller H . Apoptosis, stem cells, and tissue regeneration. Sci Signal 2010; 3: re8.
Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD et al. Compensatory proliferation and apoptosis-induced proliferation: a need for clarification. Cell Death Differ 2012; e-pub ahead of print 22 June 2012; doi:10.1038/cdd.2012.82.
Morata G, Shlevkov E, Perez-Garijo A . Mitogenic signaling from apoptotic cells in Drosophila. Dev Growth Differ 2011; 53: 168–176.
Ryoo HD, Gorenc T, Steller H . Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 2004; 7: 491–501.
Perez-Garijo A, Shlevkov E, Morata G . The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development 2009; 136: 1169–1177.
Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M . DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol 2006; 26: 7258–7268.
Wells BS, Yoshida E, Johnston LA . Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol 2006; 16: 1606–1615.
Fan Y, Bergmann A . Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell!. Trends Cell Biol 2008; 18: 467–473.
Chera S, Ghila L, Wenger Y, Galliot B . Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ 2011; 53: 186–201.
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 2011; 17: 860–866.
Valentin-Vega YA, Okano H, Lozano G . The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ 2008; 15: 1772–1781.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Green DR, Kroemer G . Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127–1130.
Rutkowski R, Hofmann K, Gartner A . Phylogeny and function of the invertebrate p53 superfamily. Cold Spring Harb Perspect Biol 2010; 2: a001131.
Lu WJ, Chapo J, Roig I, Abrams JM . Meiotic recombination provokes functional activation of the p53 regulatory network. Science 2010; 328: 1278–1281.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G . The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol 2011; 12: 259–265.
Dotsch V, Bernassola F, Coutandin D, Candi E, Melino G . p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol 2010; 2: a004887.
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev 2005; 19: 2122–2137.
Marcel V, Dichtel-Danjoy ML, Sagne C, Hafsi H, Ma D, Ortiz-Cuaran S et al. Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ 2011; 18: 1815–1824.
Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 2000; 101: 91–101.
Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM . Drosophila p53 binds a damage response element at the reaper locus. Cell 2000; 101: 103–113.
Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A et al. Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci USA 2000; 97: 7301–7306.
McEwen DG, Peifer M . Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development 2005; 132: 3935–3946.
Yamada Y, Davis KD, Coffman CR . Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the outsiders monocarboxylate transporter. Development 2008; 135: 207–216.
Fan Y, Lee TV, Xu D, Chen Z, Lamblin AF, Steller H et al. Dual roles of Drosophila p53 in cell death and cell differentiation. Cell Death Differ 2010; 17: 912–921.
Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy ML et al. ER stress protects from retinal degeneration. EMBO J 2009; 28: 1296–1307.
Jassim OW, Fink JL, Cagan RL . Dmp53 protects the Drosophila retina during a developmentally regulated DNA damage response. EMBO J 2003; 22: 5622–5632.
Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG et al. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 2004; 24: 1219–1231.
Mandal S, Freije WA, Guptan P, Banerjee U . Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J Cell Biol 2010; 188: 473–479.
Biteau B, Jasper H . It’s all about balance: p53 and aging. Aging (Albany NY) 2009; 1: 884–886.
Fish MP, Groth AC, Calos MP, Nusse R . Creating transgenic Drosophila by microinjecting the site-specific phiC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protoc 2007; 2: 2325–2331.
Guillen I, Mullor JL, Capdevila J, Sanchez-Herrero E, Morata G, Guerrero I . The function of engrailed and the specification of Drosophila wing pattern. Development 1995; 121: 3447–3456.
Swanhart LM, Sanders AN, Duronio RJ . Normal regulation of Rbf1/E2f1 target genes in Drosophila type 1 protein phosphatase mutants. Dev Dyn 2007; 236: 2567–2577.
Shlevkov E, Morata G . A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ 2012; 19: 457–460.
Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev 2002; 16: 1568–1581.
Perez-Garijo A, Martin FA, Morata G . Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development 2004; 131: 5591–5598.
Huh JR, Guo M, Hay BA . Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol 2004; 14: 1262–1266.
Fan Y, Bergmann A . Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell 2008; 14: 399–410.
Suissa Y, Ziv O, Dinur T, Arama E, Gerlitz O . The NAB-Brk signal bifurcates at JNK to independently induce apoptosis and compensatory proliferation. J Biol Chem 2011; 286: 15556–15564.
Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 2002; 21: 6722–6728.
Aoubala M, Murray-Zmijewski F, Khoury MP, Fernandes K, Perrier S, Bernard H et al. p53 directly transactivates Delta133p53alpha, regulating cell fate outcome in response to DNA damage. Cell Death Differ 2011; 18: 248–258.
Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP et al. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev 2009; 23: 278–290.
Mollereau B, Wernet MF, Beaufils P, Killian D, Pichaud F, Kuhnlein R et al. A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech Dev 2000; 93: 151–160.
Moon NS, Di Stefano L, Morris EJ, Patel R, White K, Dyson NJ . E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet 2008; 4: e1000153.
White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H . Genetic control of programmed cell death in Drosophila. Science 1994; 264: 677–683.
Acknowledgements
This work was supported by grant from the CNRS (ATIP) and Ligue contre le cancer (Comités de Savoie and Puy-de-Dôme) to BM and NIH R01GM079425 to HDR. Bench fees were funded by a Marie Curie Fellowship ERG (PERG03-GA-2008-230812) to MLDD. FN was supported by a fellowship of the Association Française contre les Myopathies. Work on p53 isoforms by PH and his team is supported by a grant form the French National Cancer Institute (INCa). This work was made possible by the DROSO-TOOLS and PLATIM facilities of the UMS3444, Biosciences, Lyon, France. We thank Virginie Marcel for critical reading of the manuscript. We also thank Carmen Garrido for technical help, and our colleagues and Bloomington centre for fly stocks and reagents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Oren
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Dichtel-Danjoy, ML., Ma, D., Dourlen, P. et al. Drosophila p53 isoforms differentially regulate apoptosis and apoptosis-induced proliferation. Cell Death Differ 20, 108–116 (2013). https://doi.org/10.1038/cdd.2012.100
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.100
Keywords
This article is cited by
-
Structural diversity of p63 and p73 isoforms
Cell Death & Differentiation (2022)
-
Srlp is crucial for the self-renewal and differentiation of germline stem cells via RpL6 signals in Drosophila testes
Cell Death & Disease (2019)
-
Drosophila jumu modulates apoptosis via a JNK-dependent pathway and is required for other processes in wing development
Apoptosis (2019)
-
A suppressive role of guanine nucleotide-binding protein subunit beta-4 inhibited by DNA methylation in the growth of anti-estrogen resistant breast cancer cells
BMC Cancer (2018)
-
p53 on the crossroad between regeneration and cancer
Cell Death & Differentiation (2017)