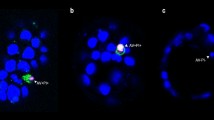
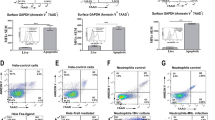
Abstract
Impaired efferocytosis has been shown to be associated with, and even to contribute to progression of, chronic inflammatory diseases such as atherosclerosis. Enhancing efferocytosis has been proposed as strategy to treat diseases involving inflammation. Here we present the strategy to increase ‘eat me’ signals on the surface of apoptotic cells by targeting cell surface-expressed phosphatidylserine (PS) with a variant of annexin A5 (Arg-Gly-Asp–annexin A5, RGD–anxA5) that has gained the function to interact with αvβ3 receptors of the phagocyte. We describe design and characterization of RGD–anxA5 and show that introduction of RGD transforms anxA5 from an inhibitor into a stimulator of efferocytosis. RGD–anxA5 enhances engulfment of apoptotic cells by phorbol-12-myristate-13-acetate-stimulated THP-1 (human acute monocytic leukemia cell line) cells in vitro and resident peritoneal mouse macrophages in vivo. In addition, RGD–anxA5 augments secretion of interleukin-10 during efferocytosis in vivo, thereby possibly adding to an anti-inflammatory environment. We conclude that targeting cell surface-expressed PS is an attractive strategy for treatment of inflammatory diseases and that the rationally designed RGD–anxA5 is a promising therapeutic agent.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
Abbreviations
- cRGD:
-
cyclic Arg-Gly-Asp
- EDTA:
-
ethylenediaminetetraacetic acid
- EGTA:
-
ethylene glycol tetraacetic acid
- IL-10:
-
interleukin-10
- MALDI-TOF/TOF:
-
matrix-assisted laser desorption/ionization-time of flight/time of flight
- MFG-E8:
-
milk fat globule-epidermal growth factor 8
- PMA:
-
phorbol-12-myristate-13-acetate
- PS:
-
phosphatidylserine
- RGD–anxA5:
-
Arg-Gly-Asp–annexin A5
- RGT–anxA5:
-
Arg-Gly-Thr–annexin A5
- THP-1:
-
human acute monocytic leukemia cell line
- TIM-4:
-
T-cell immunoglobulin- and mucin-domain-containing molecule-4
- TNFα:
-
tumor necrosis factorα
References
Krysko DV, D’Herde K, Vandenabeele P . Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 2006; 11: 1709–1726.
Savill J, Dransfield I, Gregory C, Haslett C . A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965–975.
Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 2007; 115: 2168–2177.
Gaipl US, Beyer TD, Baumann I, Voll RE, Stach CM, Heyder P et al. Exposure of anionic phospholipids serves as anti-inflammatory and immunosuppressive signal – implications for antiphospholipid syndrome and systemic lupus erythematosus. Immunobiology 2003; 207: 73–81.
Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004; 304: 1147–1150.
Tabas I . Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol 2005; 25: 2255–2264.
Nakanishi Y, Henson PM, Shiratsuchi A . Pattern recognition in phagocytic clearance of altered self. Adv Exp Med Biol 2009; 653: 129–138.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM . Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148: 2207–2216.
Ravichandran KS . Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity 2011; 35: 445–455.
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med 2005; 46 (12): 2035–2050.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C . A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39–51.
Bondanza A, Zimmermann VS, Rovere-Querini P, Turnay J, Dumitriu IE, Stach CM et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med 2004; 200: 1157–1165.
Kenis H, van Genderen H, Deckers NM, Lux PA, Hofstra L, Narula J et al. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp Cell Res 2006; 312: 719–726.
Toda S, Hanayama R, Nagata S . Two-step engulfment of apoptotic cells. Mol Cell Biol 2012; 32: 118–125.
Mira JP, Dubois T, Oudinet JP, Lukowski S, Russo-Marie F, Geny B . Inhibition of cytosolic phospholipase A2 by annexin V in differentiated permeabilized HL-60 cells. Evidence of crucial importance of domain I type II Ca2+-binding site in the mechanism of inhibition. J Biol Chem 1997; 272: 10474–10482.
Prieto J, Eklund A, Patarroyo M . Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 1994; 156: 191–211.
Ma Y, Wong K . Reassociation and translocation of glycoprotein IIB-IIIA in EDTA-treated human platelets. Platelets 2007; 18: 451–459.
Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT . Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 2000; 39: 6200–6206.
Hato T, Pampori N, Shattil SJ . Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIbβ3. J Cell Biol 1998; 141: 1685–1695.
Kurosaka K, Watanabe N, Kobayashi Y . Production of proinflammatory cytokines by phorbol myristate acetate-treated THP-1 cells and monocyte-derived macrophages after phagocytosis of apoptotic CTLL-2 cells. J Immunol 1998; 161: 6245–6249.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM . Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998; 101: 890–898.
Kenis H, Zandbergen HR, Hofstra L, Petrov AD, Dumont EA, Blankenberg FD et al. Annexin a5 uptake in ischemic myocardium: demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J Nucl Med 2010; 51: 259–267.
Hodrea J, Majai G, Doro Z, Zahuczky G, Pap A, Rajnavolgyi E et al. The glucocorticoid dexamethasone programs human dendritic cells for enhanced phagocytosis of apoptotic neutrophils and inflammatory response. J Leukoc Biol 2011; 91: 127–136.
Chvanov M, Petersen OH, Tepikin AV . Pharmacologically directed cell disposal: labeling damaged cells for phagocytosis as a strategy against acute pancreatitis. Mol Interv 2010; 10: 80–85.
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182: 1545–1556.
Henson PM, Bratton DL, Fadok VA . The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol 2001; 2: 627–633.
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S . Identification of Tim4 as a phosphatidylserine receptor. Nature 2007; 450: 435–439.
Park D, Hochreiter-Hufford A, Ravichandran KS . The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr Biol 2009; 19: 346–351.
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S . Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417: 182–187.
Huber R, Romisch J, Paques EP . The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J 1990; 9: 3867–3874.
Ungethum L, Kenis H, Nicolaes GA, Autin L, Stoilova-McPhie S, Reutelingsperger CP . Engineered annexin A5 variants with impaired cell entry for molecular imaging of apoptosis using pretargeting strategies. J Biol Chem 2011; 286: 1903–1910.
Huber R, Schneider M, Mayr I, Romisch J, Paques EP . The calcium binding sites in human annexin V by crystal structure analysis at 2.0 A resolution. Implications for membrane binding and calcium channel activity. FEBS Lett 1990; 275: 15–21.
Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H et al. Radiolabeled alpha(v)beta3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med 1999; 40: 1061–1071.
Van Vre EA, Ait-Oufella H, Tedgui A, Mallat Z . Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 887–893.
Kurihara Y, Nakahara T, Furue M . alphaVbeta3-integrin expression through ERK activation mediates cell attachment and is necessary for production of tumor necrosis factor-α in monocytic THP-1 cells stimulated by phorbol myristate acetate. Cell Immunol 2011; 270: 25–31.
Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire-de-Lima CG, Ribeiro-Gomes FL et al. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol 2010; 185: 2044–2050.
Michlewska S, Dransfield I, Megson IL, Rossi AG . Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-α. FASEB J 2009; 23: 844–854.
Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol 1992; 149: 4029–4035.
Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S et al. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med 2004; 200: 459–467.
Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med 2004; 350: 1472–1473.
Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab 2010; 12: 142–153.
Cuypers PA, Corsel JW, Janssen MP, Kop JM, Hermens WT, Hemker HC . The adsorption of prothrombin to phosphatidylserine multilayers quantitated by ellipsometry. J Biol Chem 1983; 258: 2426–2431.
Douma K, Megens RT, Reitsma S, Prinzen L, Slaaf DW, Van Zandvoort MA . Two-photon lifetime imaging of fluorescent probes in intact blood vessels: a window to sub-cellular structural information and binding status. Microsc Res Tech 2007; 70: 467–475.
Atienza JM, Yu N, Kirstein SL, Xi B, Wang X, Xu X et al. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol 2006; 4: 597–607.
Dumont EA, Reutelingsperger CP, Smits JF, Daemen MJ, Doevendans PA, Wellens HJ et al. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med 2001; 7: 1352–1355.
Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M . The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol 2011; 179: 259–269.
Engelberts I, Moller A, Schoen GJ, van der Linden CJ, Buurman WA . Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res 1991; 10: 69–76.
Acknowledgements
This study is supported in part by the European Union grant Euregional PACT II from the Interreg IV program of Grensregio Vlaanderen-Nederland (IVA-VLANED-1.20) and by project grants from the Fund for Scientific Research Flanders (FWO-Vlaanderen, G.0728.10, G067512N to DVK) and Methusalem grant (BOF09/01M00709 to PV) from the Flemish Government. DVK is a postdoctoral fellow paid by fellowship from FWO-Vlaanderen. We are indebted to Nicole Bitsch for her technical assistance and to Dr. Gerry Nicolaes (Department Biochemistry, Molecular Modeling and Structure Analysis, Maastricht University) for preparing Figures 1a and b. Lactadherin was obtained as a kind gift from Dr. J Rasmussen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Piacentini
Supplementary information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Schutters, K., Kusters, D., Chatrou, M. et al. Cell surface-expressed phosphatidylserine as therapeutic target to enhance phagocytosis of apoptotic cells. Cell Death Differ 20, 49–56 (2013). https://doi.org/10.1038/cdd.2012.107
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.107
Keywords
This article is cited by
-
Vascular lipidomics analysis reveales increased levels of phosphocholine and lysophosphocholine in atherosclerotic mice
Nutrition & Metabolism (2023)
-
BMS794833 inhibits macrophage efferocytosis by directly binding to MERTK and inhibiting its activity
Experimental & Molecular Medicine (2022)
-
Senescence under appraisal: hopes and challenges revisited
Cellular and Molecular Life Sciences (2021)
-
AnnexinA5-pHrodo: a new molecular probe for measuring efferocytosis
Scientific Reports (2018)
-
Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis
Scientific Reports (2017)