Abstract
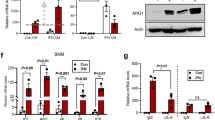
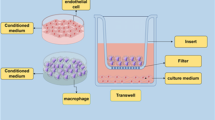
The multiple isoforms of p73, a member of the p53 family, share the ability to modulate p53 activities but also have unique properties, leading to a complex and poorly understood functional network. In vivo, p73 isoforms have been implicated in tumor suppression (TAp73−/− mice), DNA damage (ΔNp73−/− mice) and development (p73−/− mice). In this study, we investigated whether TAp73 contributes to innate immunity and septic shock. In response to a lethal lipopolysaccharide (LPS) challenge, TAp73−/− mice showed higher blood levels of proinflammatory cytokines and greater mortality than their wild-type littermates. In vitro, TAp73−/− macrophages exhibited elevated production of tumor necrosis factor alpha , interleukin-6 and macrophage inflammatory protein-2 as well as prolonged survival, decreased phagocytosis and increased major histocompatibility complex class II expression. Mice depleted of endogenous macrophages and reconstituted with TAp73−/− macrophages showed increased sensitivity to LPS challenge. These results suggest that macrophage polarization is altered in the absence of TAp73 such that maintenance of the M1 effector phenotype is prolonged at the expense of the M2 phenotype, thus impairing resolution of the inflammatory response. Our data indicate that TAp73 has a role in macrophage polarization and innate immunity, enhancing the action field of this important regulatory molecule.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- LPS:
-
lipopolysaccharide
- TNFα:
-
tumor necrosis factor alpha
- IL:
-
interleukin
- MIP:
-
macrophage inflammatory protein
- MHC:
-
major histocompatibility complex
- TLR:
-
Toll-like receptor
- i.p.:
-
intraperitoneal
- EPM:
-
elicited peritoneal macrophage
- IFN:
-
interferon
- p53RE:
-
p53-responsive element
- CXCL:
-
chemokine
- DC:
-
dendritic cell
- MEF:
-
mouse embryonic fibroblast
- UTR:
-
untranslated region
References
Yang A, Kaghad M, Caput D, McKeon F . On the shoulders of giants: p63, p73 and the raise of p53. Trends genet 2002; 18: 90–95.
Melino G, Lu X, Gasco M, Crook T, Knight RA . Functional regulation of p73 and p63: development and cancer. Trends Biochem Sci 2003; 28: 663–670.
Tomasini R, Tsushihara K, Wilhelm M, Fujitani M, Rufini A, Cheung CC et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 2008; 19: 2677–2691.
Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 2005; 4: 363–373.
Pietsch MC, Sykes SM, McMahon SB, Murphy ME . The p53 family and programmed cell death. Oncogene 2008; 27: 6505–6521.
Talos F, Nemajerova A, Flores ER, Petrenko O, Moll UM . p73 suppresses polyploidy and aneuploidy in the absence of functional p53. Mol Cell 2007; 4: 647–659.
Tomasini R, Mak TW, Melino G . The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol 2008; 5: 244–252.
Tomasini R, Tsuchihara K, Tsuda C, Lau SK, Wilhelm M, Rufini A et al. TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc Natl Acad Sci USA 2009; 3: 797–802.
Wilhelm MT, Rufini A, Wetzel MK, Tsuchihara K, Inoue S, Tomasini R et al. Isoform-specific p73 knockout mice reveal a novel role for deltaNp73 in the DNA damage response pathway. Genes Dev 2010; 24: 549–560.
Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 2000; 404: 99–103.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G . The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol 2011; 12: 259–265.
Takeda K, Akira S . Toll-like receptors in innate immunity. Int Immunol 2005; 1: 1–14.
Lin WJ, Yeh WC . Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock 2005; 3: 206–209.
Kawai T, Akira S . TLR signaling. Cell Death Differ 2005; 13: 816–825.
Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q et al. LPS-induced down-regulation of signal regulatory protein {alpha}22. contributes to innate immune activation in macrophages. J Exp Med 2007; 11: 2719–2731.
Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization. Front Biosci 2008; 13: 453–461.
Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol 2009; 182: 4415–4422.
Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K et al. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology 2007; 46: 1564–1573.
Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG et al. p53 is a suppressor of inflammatory response in mice. FASEB J 2005; 19: 1030–1032.
Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K et al. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol 2009; 29: 6391–6400.
Mehta A, Brewington R, Chatterji M, Zoubine M, Kinasewitz GT, Peer GT et al. Infection-induced modulation of m1 and m2 phenotypes in circulating monocytes: role in immune monitoring and early prognosis of sepsis. Shock 2004; 22: 423–430.
Schroder K, Hertzog PJ, Ravasi T, Hume DA . Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75: 163–189.
Yang CP, Hayashida T, Forster N, Li C, Shen D, Maheswaran S et al. The integrin {alpha}v{beta}3-5 ligand MFG-E8 is a p63/p73 target gene in triple negative breast cancers but exhibits suppressive functions in ER+ and erbB2+ breast cancers. Cancer Res 2011; 71: 937–945.
Desvergne B . PPArdelta/beta:the lobbyist switching macrophage allegiance in favor of metabolism. Cell Metab 2008; 7: 467–469.
Sbisa E, Catalano D, Grillo G, Licciulli F, Turi A, Liuni S et al. p53FamTaG: a database resource of human p53, p63 and p73 direct target genes combining in silico prediction and microarray data. BMC Bioinform 2007; 8 (Suppl 1): S20.
Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol 2002; 3: 392–398.
Coelho LF, Magno de Freitas Almeida G, Mennechet FJ, Blangy A, Interferon-alpha Uzé G. . and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc Natl Acad Sci USA 2005; 102: 11917–11922.
Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 2010; 40: 2296–2307.
Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity 2005; 23: 319–329.
Medzhitov R . Origin and physiological roles of inflammation. Nature 2008; 454: 428–435.
De las Heras B, Hortelano S, Giron N, Bermejo P, Rodriguez B, Bosca L . Kaurane diterpenes protect against apoptosis and inhibition of phagocytosis in activated macrophages. Br J Pharmacol 2007; 2: 249–255.
Seitz SJ, Schleithoff ES, Koch A, Schuster A, Teufel A, Staib F et al. Chemotherapy-induced apoptosis in hepatocellular carcinoma involves the p53 family and is mediated via the extrinsic and the intrinsic pathway. Int J Cancer 2010; 26: 2049–2066.
Fuhrman LE, Goel AK, Smith J, Shianna KV, Aballay A . Nucleolar proteins suppress Caenorhabditis elegans innate immunity by inhibiting p53/CEP-1. PLoS Genet 2009; 5: e1000657.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356: 215–221.
Rye MS, Bhutta MF, Cheeseman MT, Burgner D, Blackwell JM, Brown SD et al. Unraveling the genetics of otitis media: from mouse to human and back again. Mamm Genome 2010; 22: 66–82.
Carrasco G, Diaz J, Valbuena JR, Ibanez P, Rodriguez P, Araya G et al. Overexpression of p73 as a tissue marker for high-risk gastritis. Clin Cancer Res 2010; 16: 3253–3259.
Murray PJ, Wynn TA . Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol 2001; 89: 557–563.
Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA et al. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol 2011; 186: 2444–2453.
Tabas I . Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 2010; 10: 36–46.
Terenzi F, Díaz-Guerra MJ, Casado M, Hortelano S, Leoni S, Boscá L . Bacterial lipopeptides induce nitric oxide synthase and promote apoptosis through nitric oxide-independent pathways in rat macrophages. J Biol Chem 1995; 270: 6017–6021.
Acknowledgements
We thank Dr. Tadatsugu Taniguchi (Department of Immunology, University of Tokyo) for kindly providing the p125 construct containing the IFNβ promoter region, and Dr. Anne Brustle for assistance and helpful discussions. We are grateful to Dr. Mary Saunders for scientific editing. We thank also Jing-Jing Liu for technical assistance. This work was supported by INSERM, and a fellowship award from l’Association pour la recherche contre le cancer (R.T) and Fondation pour la Recherche Medicale (L.P).
Author contributions
RT, GM, TWM and JLI designed the research; RT, VS, LP, AKT, JN and MW performed the research; RT, LP, VS, SV, MW, PB, GM and JLI analyzed the data; and RT wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Tomasini, R., Secq, V., Pouyet, L. et al. TAp73 is required for macrophage-mediated innate immunity and the resolution of inflammatory responses. Cell Death Differ 20, 293–301 (2013). https://doi.org/10.1038/cdd.2012.123
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.123
Keywords
This article is cited by
-
p73 isoforms meet evolution of metastasis
Cancer and Metastasis Reviews (2022)
-
Non-oncogenic roles of TAp73: from multiciliogenesis to metabolism
Cell Death & Differentiation (2018)
-
TAp73 loss favors Smad-independent TGF-β signaling that drives EMT in pancreatic ductal adenocarcinoma
Cell Death & Differentiation (2016)
-
Resolution of inflammation: a new therapeutic frontier
Nature Reviews Drug Discovery (2016)
-
How Does p73 Cause Neuronal Defects?
Molecular Neurobiology (2016)