Abstract
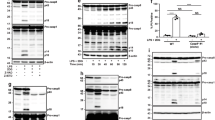
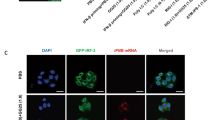
Toll-like receptor 3 (TLR3) is a pattern-recognition receptor known to initiate an innate immune response when stimulated by double-stranded RNA (dsRNA). Components of TLR3 signaling, including TIR domain-containing adapter inducing IFN-α (TRIF), have been demonstrated to contribute to dsRNA-induced cell death through caspase-8 and receptor interacting protein (RIP)1 in various human cancer cells. We provide here a detailed analysis of the caspase-8 activating machinery triggered in response to Poly(I:C) dsRNA. Engagement of TLR3 by dsRNA in both type I and type II lung cancer cells induces the formation of an atypical caspase-8-containing complex that is devoid of classical death receptors of the TNFR superfamily, but instead is physically associated to TLR3. The recruitment of caspase-8 to TLR3 requires RIP1, and is negatively modulated by cellular inhibitor of apoptosis protein (cIAP)2–TNF receptor-associated factor (TRAF)2–TNFR-associated death domain (TRADD) ubiquitin ligase complex, which regulates RIP1 ubiquitination. Intriguingly, unlike Fas- or TRAILR-dependent death signaling, caspase-8 recruitment and activation within the TLR3 death-signaling complex appears not to be stringently dependent on Fas-associated with death domain (FADD). Our findings uncover a novel aspect of the molecular mechanisms involved during apoptosis induced by the innate immune receptor TLR3 in cancer cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DD:
-
death domain
- DISC:
-
death-inducing signaling complex
- DR:
-
death receptor
- dsRNA:
-
double-stranded RNA
- FADD:
-
Fas-associated with death domain
- FLIPL:
-
FLICE-like inhibitory protein
- ISRE:
-
interferon-stimulated response element
- MDA5:
-
melanoma differentiation-associated protein 5
- NSCLC:
-
non small cell lung cancer
- PI:
-
propidium iodide
- PKR:
-
protein kinase RNA-activated
- RHIM:
-
RIP homotypic interaction motif
- RIG-I:
-
retinoic acid-inducible gene I protein
- RIP:
-
receptor interacting protein
- Ter:
-
terminal
- TIR:
-
Toll-IL-1-receptor
- TLR:
-
Toll-like receptor
- TRADD:
-
TNFR-associated death domain
- TRAF:
-
TNF receptor-associated factor
- TRIF:
-
TIR domain-containing adapter inducing IFN-α
References
Levy HB, Law LW, Rabson AS . Inhibition of tumor growth by polyinosinic-polycytidylic acid. Proc Natl Acad Sci USA 1969; 62: 357–361.
Kalai M, Van Loo G, Vanden Berghe T, Meeus A, Burm W, Saelens X et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ 2002; 9: 981–994.
Takeuchi O, Akira S . Innate immunity to virus infection. Immunol Rev 2009; 227: 75–86.
Funami K, Sasai M, Ohba Y, Oshiumi H, Seya T, Matsumoto M . Spatiotemporal mobilization of Toll/IL-1 receptor domain-containing adaptor molecule-1 in response to dsRNA. J Immunol 2007; 179: 6867–6872.
Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T . TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 2006; 176: 4894–4901.
Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P . Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res 2007; 13: 4565–4574.
Friboulet L, Pioche-Durieu C, Rodriguez S, Valent A, Souquere S, Ripoche H et al. Recurrent overexpression of c-IAP2 in EBV-associated nasopharyngeal carcinomas: critical role in resistance to Toll-like receptor 3-mediated apoptosis. Neoplasia 2008; 10: 1183–1194.
Salaun B, Zitvogel L, Asselin-Paturel C, Morel Y, Chemin K, Dubois C et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res 2011; 71: 1607–1614.
Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Hacker G . Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ 2009; 17: 942–951.
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M et al. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011; 43: 449–463.
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 2004; 5: 503–507.
Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Hacker G et al. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol 2004; 173: 3320–3328.
Kaiser WJ, Offermann MK . Apoptosis induced by the Toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 2005; 174: 4942–4952.
Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 2011; 43: 432–448.
Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA et al. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 2011; 34: 866–878.
Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB . VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 2005; 19: 727–740.
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–321.
Scaffidi C, Schmitz I, Krammer PH, Peter ME . The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 1999; 274: 1541–1548.
Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem 2002; 277: 45162–45171.
Rebsamen M, Meylan E, Curran J, Tschopp J . The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ 2008; 15: 1804–1811.
Lin Y, Devin A, Rodriguez Y, Liu ZG . Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 1999; 13: 2514–2526.
Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C . Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 2003; 18: 655–664.
Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ . Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 2006; 22: 245–257.
Micheau O, Tschopp J . Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003; 114: 181–190.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT . Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol 2007; 17: 418–424.
Wang L, Du F, Wang X . TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703.
O’Donnell MA, Ting AT . RIP1 comes back to life as a cell death regulator in TNFR1 signaling. FEBS J 2011; 278: 877–887.
Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem 2008; 283: 24295–24299.
Ermolaeva MA, Michallet MC, Papadopoulou N, Utermohlen O, Kranidioti K, Kollias G et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol 2008; 9: 1037–1046.
Sasai M, Tatematsu M, Oshiumi H, Funami K, Matsumoto M, Hatakeyama S et al. Direct binding of TRAF2 and TRAF6 to TICAM-1/TRIF adaptor participates in activation of the Toll-like receptor 3/4 pathway. Mol Immunol 2010; 47: 1283–1291.
Tao S, Zhu L, Lee P, Lee WM, Knox K, Chen J et al. Negative control of TLR3 signaling by TICAM1 downregulation. Am J Respir Cell Mol Biol 2011; e-pub ahead of print 28 December 2011.
Knox PG, Davies CC, Ioannou M, Eliopoulos AG . The death domain kinase RIP1 links the immunoregulatory CD40 receptor to apoptotic signaling in carcinomas. J Cell Biol 2011; 192: 391–399.
Shikama Y, Yamada M, Miyashita T . Caspase-8 and caspase-10 activate NF-kappaB through RIP, NIK and IKKalpha kinases. Eur J Immunol 2003; 33: 1998–2006.
Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J . NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 2001; 21: 5299–5305.
Upton JW, Kaiser WJ, Mocarski ES . Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem 2008; 283: 16966–16970.
Dufour F, Bertrand L, Pearson A, Grandvaux N, Langelier Y . The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against poly(I:C)-induced apoptosis. J Virol 2011; 85: 8689–8701.
Salaun B, Romero P, Lebecque S . Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur J Immunol 2007; 37: 3311–3318.
Maelfait J, Beyaert R . Non-apoptotic functions of caspase-8. Biochem Pharmacol 2008; 76: 1365–1373.
Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM . Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med 2011; 208: 643–651.
Acknowledgements
We thank our colleagues who provided reagents, especially Patrick Mehlen for the Bcl-2-expressing plasmid, Wayne Fairbrother for the gift of Smac mimetic BV6, Isabelle Grosjean for her assistance with cultures, Stéphanie Brunet-Manquat and Isabelle Durand for their support with flow cytometry, Jean-Paul Pais De Barros for assistance with size-exclusion chromatography, and our colleagues in the laboratory for their restless support and encouragement. The Cancéropole Lyon Auvergne Rhone-Alpes (CLARA) funded the work and SL was supported by a grant from the Hospices Civils de Lyon. OM was supported by grants from the ANR (Agence Nationale de la Recherche 07-PCV-0031) and the European Community (ApopTrain Marie Curie RTN). GJ and NL were supported by fellowships from the Ligue Nationale contre le Cancer and a joint INSERM and Conseil Régional de Bourgogne fellowship, respectively. We gratefully acknowledge the help and advice of Nicolas Foray.
Author Contributions
YE, FT, FV, OM, and SL conceived and designed the experiments. YE, FT, FV, GJ, MB, AP, BV, NL, and PM-G performed the experiments and data analysis. TR, BS, and YP contributed to data analysis, discussions, and intellectual input. YE, FT, FV, OM, and SL wrote the paper with all authors providing detailed comments and suggestions. SL directed the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by J Silke
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Estornes, Y., Toscano, F., Virard, F. et al. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ 19, 1482–1494 (2012). https://doi.org/10.1038/cdd.2012.22
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.22
Keywords
This article is cited by
-
The TLR3 L412F polymorphism prevents TLR3-mediated tumor cell death induction in pediatric sarcomas
Cell Death Discovery (2023)
-
Emerging role of RNA sensors in tumor microenvironment and immunotherapy
Journal of Hematology & Oncology (2022)
-
PKR and TLR3 trigger distinct signals that coordinate the induction of antiviral apoptosis
Cell Death & Disease (2022)
-
Immunotherapeutic role of cabazitaxel treatment in the activation of TLR3 signalling in metastatic castration-resistant prostate cancer in vitro
Molecular Biology Reports (2022)
-
Anti-viral and pro-inflammatory functions of Toll-like receptors during gamma-herpesvirus infections
Virology Journal (2021)