Abstract
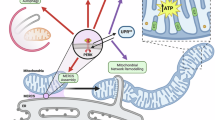
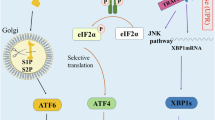
Endoplasmic reticulum stress is emerging as an important modulator of different pathologies and as a mechanism contributing to cancer cell death in response to therapeutic agents. In several instances, oxidative stress and the onset of endoplasmic reticulum (ER) stress occur together; yet, the molecular events linking reactive oxygen species (ROS) to ER stress-mediated apoptosis are currently unknown. Here, we show that PERK (RNA-dependent protein kinase (PKR)-like ER kinase), a key ER stress sensor of the unfolded protein response, is uniquely enriched at the mitochondria-associated ER membranes (MAMs). PERK−/− cells display disturbed ER morphology and Ca2+ signaling as well as significantly weaker ER-mitochondria contact sites. Re-expression of a kinase-dead PERK mutant but not the cytoplasmic deletion mutant of PERK in PERK−/− cells re-establishes ER-mitochondria juxtapositions and mitochondrial sensitization to ROS-mediated stress. In contrast to the canonical ER stressor thapsigargin, during ROS-mediated ER stress, PERK contributes to apoptosis twofold by sustaining the levels of pro-apoptotic C/EBP homologous protein (CHOP) and by facilitating the propagation of ROS signals between the ER and mitochondria through its tethering function. Hence, this study reveals an unprecedented role of PERK as a MAMs component required to maintain the ER-mitochondria juxtapositions and propel ROS-mediated mitochondrial apoptosis. Furthermore, it suggests that loss of PERK may cause defects in cell death sensitivity in pathological conditions linked to ROS-mediated ER stress.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CHOP:
-
C/EBP homologous protein
- eIF2α:
-
eukaryotic initiation factor-2α
- ER:
-
endoplasmic reticulum
- GRP78:
-
glucose regulated protein 78
- IP3R:
-
inositol 1,4,5-trisphosphate receptor
- IRE1:
-
inositol requiring enzyme 1
- JNK:
-
c-Jun N-terminal kinase
- MAM:
-
mitochondria-associated ER membrane
- MEF:
-
murine embryonic fibroblast
- MFN2:
-
mitofusin 2
- PERK:
-
RNA-dependent protein kinase (PKR)-like ER kinase
- Phox:
-
photo-oxidative
- ROS:
-
reactive oxygen species
- SERCA:
-
sarco/endoplasmic reticulum Ca2+ ATPase
- TG:
-
thapsigargin
- UPR:
-
unfolded protein response
References
Malhotra JD, Kaufman RJ . The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 2007; 18: 716–731.
Tabas I, Ron D . Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011; 13: 184–190.
Verfaillie T, Garg AD, Agostinis P . Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 2010 e-pub ahead of print 21 August 2010; doi: 10.1016/j.canlet.2010.07.016.
Malhotra JD, Kaufman RJ . Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 2007; 9: 2277–2293.
Pinton P, Giorgi C, Pandolfi PP . The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ 2011; 18: 1450–1456.
Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 2009; 36: 500–511.
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011; 61: 250–281.
Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L et al. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J 2006; 20: 756–758.
Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31: 1062–1079.
Buytaert E, Matroule JY, Durinck S, Close P, Kocanova S, Vandenheede JR et al. Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene 2008; 27: 1916–1929.
Hayashi T, Rizzuto R, Hajnoczky G, Su TP . MAM: more than just a housekeeper. Trends Cell Biol 2009; 19: 81–88.
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 2007; 13: 365–376.
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11: 619–633.
Yamaguchi Y, Larkin D, Lara-Lemus R, Ramos-Castaneda J, Liu M, Arvan P . Endoplasmic reticulum (ER) chaperone regulation and survival of cells compensating for deficiency in the ER stress response kinase, PERK. J Biol Chem 2008; 283: 17020–17029.
Redondo PC, Salido GM, Rosado JA, Pariente JA . Effect of hydrogen peroxide on Ca2+ mobilisation in human platelets through sulphydryl oxidation dependent and independent mechanisms. Biochem Pharmacol 2004; 67: 491–502.
Wei H, Li Z, Hu S, Chen X, Cong X . Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem 2010; 111: 967–978.
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D . Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 2000; 5: 897–904.
Novosyadlyy R, Kurshan N, Lann D, Vijayakumar A, Yakar S, LeRoith D . Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ 2008; 15: 1304–1317.
Huang G, Yao J, Zeng W, Mizuno Y, Kamm KE, Stull JT et al. ER stress disrupts Ca2+-signaling complexes and Ca2+ regulation in secretory and muscle cells from PERK-knockout mice. J Cell Sci 2006; 119 (Part 1): 153–161.
Hogan PG, Lewis RS, Rao A . Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol 2010; 28: 491–533.
Hendrickx N, Dewaele M, Buytaert E, Marsboom G, Janssens S, Van Boven M et al. Targeted inhibition of p38alpha MAPK suppresses tumor-associated endothelial cell migration in response to hypericin-based photodynamic therapy. Biochem Biophys Res Commun 2005; 337: 928–935.
Agarwal ML, Larkin HE, Zaidi SI, Mukhtar H, Oleinick NL . Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma cells. Cancer Res 1993; 53: 5897–5902.
Orrenius S, Zhivotovsky B . Cardiolipin oxidation sets cytochrome c free. Nat Chem Biol 2005; 1: 188–189.
Korytowski W, Basova LV, Pilat A, Kernstock RM, Girotti AW . Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis. J Biol Chem 2011; 286: 26334–26343.
Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T . Mitochondria as biosensors of calcium microdomains. Cell Calcium 1999; 26: 193–199.
Brough D, Schell MJ, Irvine RF . Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J 2005; 392 (Part 2): 291–297.
Harding HP, Zhang Y, Ron D . Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397: 271–274.
de Brito OM, Scorrano L . Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008; 456: 605–610.
Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA . Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 2003; 23: 7198–7209.
Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 2010; 29: 3881–3895.
Gilady SY, Bui M, Lynes EM, Benson MD, Watts R, Vance JE et al. Ero1alpha requires oxidizing and normoxic conditions to localize to the mitochondria-associated membrane (MAM). Cell Stress Chaperones 2010; 15: 619–629.
Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A et al. Ero1alpha regulates Ca(2+) fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal 2012; 15: 1077–1087.
Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P . Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 2008; 32: 641–651.
Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 2011; 31: 1309–1328.
Cerqua C, Anesti V, Pyakurel A, Liu D, Naon D, Wiche G et al. Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition. EMBO Rep 2010; 11: 854–860.
Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 2010; 330: 1247–1251.
Vantieghem A, Assefa Z, Vandenabeele P, Declercq W, Courtois S, Vandenheede JR et al. Hypericin-induced photosensitization of HeLa cells leads to apoptosis or necrosis. Involvement of cytochrome c and procaspase-3 activation in the mechanism of apoptosis. FEBS Lett 1998; 440: 19–24.
Delaey EM, Vandenbogaerde AL, Agostinis P, De Witte PA . Confluence dependent resistance to photo-activated hypericin in HeLa cells. Int J Oncol 1999; 14: 759–763.
Missiaen L, Parys JB, Weidema AF, Sipma H, Vanlingen S, De Smet P et al. The bell-shaped Ca2+ dependence of the inositol 1,4,5-trisphosphate-induced Ca2+ release is modulated by Ca2+/calmodulin. J Biol Chem 1999; 274: 13748–13751.
Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB . The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta 2011; 1813: 1003–1013.
Acknowledgements
This work is supported by GOA grant of the KU Leuven and by F.W.O grant G.0492.05 to PA, G.0788.11N, and OTSRT1/10/44 to GB. This paper presents research results of the IAP6/18 and IAP6/28, funded by the Interuniversity Attraction Poles Programme, initiated by the Belgian State, Science Policy Office. NR is a post-doctoral fellow supported by F.R.S-FNRS (grant F/5/4/5-MCF/KP). J-PD is a recipient of a PhD Fellowship from the Agency for Innovation of Science and Technology (IWT). We thank Dr. R Kaufman (University of Michigan Medical Center, Ann Arbor, MI, USA) for the WT, PERK−/−, CHOP−/− and IRE1−/− fibroblasts, Dr. A Diehl (Abramson Family Cancer Research Institute Philadelphia, USA) for the PERK-shRNA transduced MDA-MB468 cells, Dr. D Krysko (University of Ghent, Belgium) for the PERK-shRNA CT26 cells and Dr. W Martinet (University of Antwerpen, Belgium) for the electron microscopy analysis. We thank Professor M Fransen for the use of the Neon Electroporation equipment. We acknowledge Dr. S Bontems for excellent support in transforming MEFs with KRAS, Tomas Luyten for excellent support in the Ca2+ experiments, Veerle Daniels for help with MACs isolation, and Jan Piessens and Kristine Rillaerts for skillful technical support. Nikon confocal microscope was acquired through a Hercules Type 1 AKUL/09/037 to W Annaert.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by SH Kaufmann
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Verfaillie, T., Rubio, N., Garg, A. et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19, 1880–1891 (2012). https://doi.org/10.1038/cdd.2012.74
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.74
Keywords
This article is cited by
-
Relevance of the endoplasmic reticulum-mitochondria axis in cancer diagnosis and therapy
Experimental & Molecular Medicine (2024)
-
Human neural stem cell secretome relieves endoplasmic reticulum stress-induced apoptosis and improves neuronal functions after traumatic brain injury in a rat model
Journal of Molecular Histology (2024)
-
Silybin attenuates avermectin-induced oxidative damage in carp respiration by modulating the cGAS-STING pathway and endoplasmic reticulum stress
Fish Physiology and Biochemistry (2024)
-
Methane-Rich Saline Suppresses ER-Mitochondria Contact and Activation of the NLRP3 Inflammasome by Regulating the PERK Signaling Pathway to Ameliorate Intestinal Ischemia‒Reperfusion Injury
Inflammation (2024)
-
Homocysteine promotes atherosclerosis through macrophage pyroptosis via endoplasmic reticulum stress and calcium disorder
Molecular Medicine (2023)