Abstract
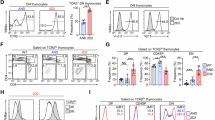
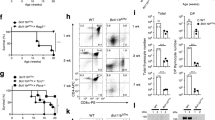
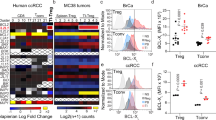
T cells developing in the thymus undergo rigorous positive and negative selection to ensure that those exported to peripheral lymphoid organs bear T-cell receptors (TCRs) capable of reacting with foreign antigens but tolerant of self. At each checkpoint, whether a thymocyte survives or dies is determined by antiapoptotic and proapoptotic Bcl-2 family members. We used Mcl-1 transgenic (tg) mice to investigate the impact of elevated expression of antiapoptotic Mcl-1 on thymocyte apoptosis and selection, making a side-by-side comparison with thymocytes from BCL-2tg mice. Mcl-1 was as effective as Bcl-2 at protecting thymocytes against spontaneous cell death, diverse cytotoxic insults and TCR–CD3 stimulation-driven apoptosis. In three different TCR tg models, Mcl-1 markedly enhanced positive selection of thymocytes, as did Bcl-2. In H-Y TCR tg mice, elevated Mcl-1 and Bcl-2 were equally effective at inhibiting deletion of autoreactive thymocytes. However, in the OT-1tg model where deletion is mediated by a peripheral antigen whose expression is regulated by Aire, Mcl-1 was less effective than Bcl-2. Thus, the capacity of Mcl-1 overexpression to inhibit apoptosis triggered by TCR stimulation apparently depends on the thymocyte subset subject to deletion, presumably due to differences in the profiles of proapoptotic Bcl-2 family members mediating the deletion.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TCR:
-
T-cell receptor
- DN:
-
double-negative
- DP:
-
double-positive
- SP:
-
single-positive
- MHC:
-
major histocompatibility complex
- PMA:
-
phorbol 12-myristate 13-acetate
- WT:
-
wild-type
- BM:
-
bone marrow
- tg:
-
transgenic
- OVA:
-
ovalbumin
- RIP:
-
rat insulin promoter
- WEHI:
-
The Walter and Eliza Hall Institute
References
Carpenter AC, Bosselut R . Decision checkpoints in the thymus. Nat Immunol 2010; 11: 666–673.
Love PE, Bhandoola A . Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol 2011; 11: 469–477.
Hogquist KA, Baldwin TA, Jameson SC . Central tolerance: learning self-control in the thymus. Nat Rev Immunol 2005; 5: 772–782.
Mathis D, Benoist C . Aire. Annu Rev Immunol 2009; 27: 287–312.
Strasser A, Cory S, Adams JM . Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J 2011; 30: 3667–3683.
Pezzella F, Tse AGD, Cordell JL, Pulford KAF, Gatter KC, Mason DY . Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol 1990; 137: 225–232.
Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ . Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol 1993; 151: 2546–2554.
Grillot DAM, Merino R, Nuñez G . Bcl-xL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med 1995; 182: 1973–1983.
Tomayko MM, Punt JA, Bolcavage JM, Levy SL, Allman DM, Cancro MP . Expression of the Bcl-2 family member A1 is developmentally regulated in T cells. Int Immunol 1999; 11: 1753–1761.
Verschelde C, Michonneau D, Trescol-Biemont MC, Berberich I, Schimpl A, Bonnefoy-Berard N . Overexpression of the antiapoptotic protein A1 promotes the survival of double positive thymocytes awaiting positive selection. Cell Death Differ 2006; 13: 1213–1221.
Dzhagalov I, Dunkle A, He YW . The anti-apoptotic bcl-2 family member mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol 2008; 181: 521–528.
Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 2002; 415: 922–926.
Villunger A, Marsden VS, Zhan Y, Erlacher M, Lew AM, Bouillet P et al. Negative selection of semimature CD4(+)8(-)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci USA 2004; 101: 7052–7057.
Strasser A, Harris AW, Cory S . Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 1991; 67: 889–899.
Strasser A, Harris AW, von Boehmer H, Cory S . Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA 1994; 91: 1376–1380.
Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ . bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 1991; 67: 879–888.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393–403.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535.
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 2005; 19: 1294–1305.
Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007; 315: 856–859.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ . Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671–676.
Dunkle A, Dzhagalov I, He YW . Mcl-1 promotes survival of thymocytes by inhibition of Bak in a pathway separate from Bcl-2. Cell Death Differ 2010; 17: 994–1002.
Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 2010; 116: 3197–3207.
Jorgensen TN, McKee A, Wang M, Kushnir E, White J, Refaeli Y et al. Bim and Bcl-2 mutually affect the expression of the other in T cells. J Immunol 2007; 179: 3417–3424.
Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C et al. Bcl-2, Bcl-xL and Bcl-w are not equivalent targets of ABT-737 and Navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816.
Strasser A . The role of BH3-only proteins in the immune system. Nat Rev. Immunol 2005; 5: 189–200.
De Biasio A, Vrana JA, Zhou P, Qian L, Bieszczad CK, Braley KE et al. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J Biol Chem 2007; 282: 23919–23936.
von Boehmer H . Developmental biology of T cells in T cell-receptor transgenic mice. Ann Rev Immunol 1990; 8: 531–556.
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM . Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 1999; 96: 14943–14948.
von Boehmer H, Kisielow P . Self-nonself discrimination by T cells. Science 1990; 248: 1369–1373.
Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D . The cellular mechanism of Aire control of T cell tolerance. Immunity 2005; 23: 227–239.
Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 2011; 118: 2462–2472.
Gui J, Morales AJ, Maxey SE, Bessette KA, Ratcliffe NR, Kelly JA et al. MCL1 increases primitive thymocyte viability in female mice and promotes thymic expansion into adulthood. Int Immunol 2011; 23: 647–659.
Yang CY, Lin NH, Lee JM, Huang CY, Min HJ, Yen JJ et al. Promoter knock-in mutations reveal a role of Mcl-1 in thymocyte-positive selection and tissue or cell lineage-specific regulation of Mcl-1 expression. J Immunol 2009; 182: 2959–2968.
Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208: 1279–1289.
Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S . Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183–192.
Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG et al. BAX activation is initiated at a novel interaction site. Nature 2008; 455: 1076–1081.
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 2009; 36: 487–499.
Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 2009; 186: 355–362.
Ogilvy S, Metcalf D, Gibson L, Bath ML, Harris AW, Adams JM . Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood 1999; 94: 1855–1863.
Acknowledgements
We thank our colleagues C Vandenberg, C Scott, K Heger and P Bouillet for useful discussions; L O’Reilly and D Huang for reagents; J Blyth and F Kupresanin for assistance; B Helbert and C Young for genotyping; K McKenzie, T Camilleri and G Siciliano for mouse husbandry; and WEHI cytometry and histology services. This work was supported by postdoctoral fellowships from EMBO and the Human Frontier in Science Program (KJC); NHMRC Career Development Fellowship (DHDG) (CDF1 #637353); NHMRC Australia Fellowship (AS); research grants from National Health and Medical Research Council (NHMRC) (programme grant 461221) and National Cancer Institute (CA43540); and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by S Nagata
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Campbell, K., Gray, D., Anstee, N. et al. Elevated Mcl-1 inhibits thymocyte apoptosis and alters thymic selection. Cell Death Differ 19, 1962–1971 (2012). https://doi.org/10.1038/cdd.2012.84
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.84
Keywords
This article is cited by
-
Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice
Cell Death & Differentiation (2019)
-
Re-activation of mitochondrial apoptosis inhibits T-cell lymphoma survival and treatment resistance
Leukemia (2016)
-
Loss of Bak enhances lymphocytosis but does not ameliorate thrombocytopaenia in BCL-2 transgenic mice
Cell Death & Differentiation (2014)