Abstract
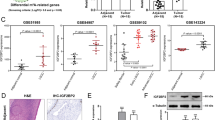
Transforming growth factor-α (TGF-α)-induced proliferation and transforming growth factor-β (TGF-β)-mediated quiescence are intricately balanced in normal lung-tissue homeostasis but are deregulated during neoplastic progression of lung cancer. Here, we show that Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2), a novel MYC-interacting transcriptional modulator, responds to TGF-α induction and TGF-β suppression to orchestrate cellular proliferation and quiescence, respectively. Upon TGF-α induction, CITED2 was induced by MYC and further modulated MYC-mediated transcription in a feed-forward manner. CITED2 recruited p300 to promote MYC-p300-mediated transactivation of E2F3, leading to increased G1/S cell cycle progression. Moreover, CITED2 inhibited cellular quiescence by enhancing MYC-mediated suppression of p21CIP1. CITED2 interacted with histone deacetylase 1 (HDAC1) and potentiated MYC–HDAC1 complex formation. TGF-β stimulation provoked downregulation of CITED2, which abrogated MYC-HDAC1-mediated p21CIP1 suppression, causing cellular quiescence. Ectopic CITED2 expression enhanced tumor growth in nude mice; furthermore, CITED2 knockdown caused tumor shrinkage and increased overall host mouse survival rates. Expression of CITED2/MYC/E2F3/p21CIP1 signaling molecules was associated with poor prognosis of lung cancer patients. Thus, CITED2 functions as a molecular switch of TGF-α and TGF-β-induced growth control, and MYC-CITED2 signaling axis provides a new index for predicting clinical outcome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CITED2:
-
Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2
- TGF-α:
-
transforming growth factor alpha
- TGF-β:
-
transforming growth factor beta
- NSCLC:
-
non-small-cell lung cancer
- EGFR:
-
epidermal growth factor receptor
- TGFBRI:
-
transforming growth factor beta receptor I
- TGFBRII:
-
transforming growth factor beta receptor II
- p300:
-
E1A binding protein p300
- HDAC1:
-
histone deacetylase 1
- Dox:
-
doxycycline
- eGFP:
-
enhanced green fluorescent protein
- shRNA:
-
short hairpin RNA
- GST:
-
glutathione S-transferase
References
Jetten AM . Growth and differentiation factors in tracheobronchial epithelium. Am J Physiol 1991; 260 (6 Pt 1): L361–L373.
Sharma SV, Bell DW, Settleman J, Haber DA . Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–181.
Rusch V, Baselga J, Cordon-Cardo C, Orazem J, Zaman M, Hoda S et al. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res 1993; 53 (10 Suppl): 2379–2385.
Antoshina E, Ostrowski LE . TGF beta 1 induces growth arrest and apoptosis but not ciliated cell differentiation in rat tracheal epithelial cell cultures. In Vitro Cell Dev Biol Anim 1997; 33: 212–217.
Zhang HT, Chen XF, Wang MH, Wang JC, Qi QY, Zhang RM et al. Defective expression of transforming growth factor beta receptor type II is associated with CpG methylated promoter in primary non-small cell lung cancer. Clin Cancer Res 2004; 10: 2359–2367.
Anumanthan G, Halder SK, Osada H, Takahashi T, Massion PP, Carbone DP et al. Restoration of TGF-beta signalling reduces tumorigenicity in human lung cancer cells. Br J Cancer 2005; 93: 1157–1167.
Sun HB, Zhu YX, Yin T, Sledge G, Yang YC . MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc Natl Acad Sci U S A 1998; 95: 13555–13560.
Xu B, Qu X, Gu S, Doughman YQ, Watanabe M, Dunwoodie SL et al. Cited2 is required for fetal lung maturation. Dev Biol 2008; 317: 95–105.
Chou YT, Yang YC . Post-transcriptional control of Cited2 by transforming growth factor beta. Regulation via Smads and Cited2 coding region. J Biol Chem 2006; 281: 18451–18462.
Glenn DJ, Maurer RA . MRG1 binds to the LIM domain of Lhx2 and may function as a coactivator to stimulate glycoprotein hormone alpha-subunit gene expression. J Biol Chem 1999; 274: 36159–36167.
Braganca J, Eloranta JJ, Bamforth SD, Ibbitt JC, Hurst HC, Bhattacharya S . Physical and functional interactions among AP-2 transcription factors, p300/CREB-binding protein, and CITED2. J Biol Chem 2003; 278: 16021–16029.
Tien ES, Davis JW, Vanden Heuvel JP . Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. J Biol Chem 2004; 279: 24053–24063.
Chou YT, Wang H, Chen Y, Danielpour D, Yang YC . Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene 2006; 25: 5547–5560.
Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM . Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev 1999; 13: 64–75.
Broers JL, Viallet J, Jensen SM, Pass H, Travis WD, Minna JD et al. Expression of c-myc in progenitor cells of the bronchopulmonary epithelium and in a large number of non-small cell lung cancers. Am J Respir Cell Mol Biol 1993; 9: 33–43.
Adams MR, Sears R, Nuckolls F, Leone G, Nevins JR . Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol Cell Biol 2000; 20: 3633–3639.
Leone G, Sears R, Huang E, Rempel R, Nuckolls F, Park CH et al. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol Cell 2001; 8: 105–113.
Cooper CS, Nicholson AG, Foster C, Dodson A, Edwards S, Fletcher A et al. Nuclear overexpression of the E2F3 transcription factor in human lung cancer. Lung Cancer 2006; 54: 155–162.
Reimer D, Hubalek M, Riedle S, Skvortsov S, Erdel M, Concin N et al. E2F3a is critically involved in epidermal growth factor receptor-directed proliferation in ovarian cancer. Cancer Res 2010; 70: 4613–4623.
Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 2001; 414: 457–462.
Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 2003; 22: 351–360.
Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F et al. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci U S A 2001; 98: 4510–4515.
Claassen GF, Hann SR . A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta -induced cell-cycle arrest. Proc Natl Acad Sci USA 2000; 97: 9498–9503.
Nass SJ, Dickson RB . Epidermal growth factor-dependent cell cycle progression is altered in mammary epithelial cells that overexpress c-myc. Clin Cancer Res 1998; 4: 1813–1822.
Chou YT, Lin HH, Lien YC, Wang YH, Hong CF, Kao YR et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res 2010; 70: 8822–8831.
Leung JY, Ehmann GL, Giangrande PH, Nevins JR . A role for Myc in facilitating transcription activation by E2F1. Oncogene 2008; 27: 4172–4179.
Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF . Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 1995; 92: 5545–5549.
Katakura Y, Nakata E, Tabira Y, Miura T, Teruya K, Tsuchiya T et al. Decreased tumorigenicity in vivo when transforming growth factor beta treatment causes cancer cell senescence. Biosci Biotechnol Biochem 2003; 67: 815–821.
Abbas T, Dutta A . p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–414.
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA . Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 2004; 101: 1241–1246.
Faiola F, Liu X, Lo S, Pan S, Zhang K, Lymar E et al. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol Cell Biol 2005; 25: 10220–10234.
Chen CR, Kang Y, Massague J . Defective repression of c-myc in breast cancer cells: a loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci USA 2001; 98: 992–999.
Kranc KR, Bamforth SD, Braganca J, Norbury C, van Lohuizen M, Bhattacharya S . Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via Ink4a/ARF. Mol Cell Biol 2003; 23: 7658–7666.
Afshari CA, Nichols MA, Xiong Y, Mudryj M . A role for a p21-E2F interaction during senescence arrest of normal human fibroblasts. Cell Growth Differ 1996; 7: 979–988.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805–816.
Hilbe W, Dirnhofer S, Greil R, Woll E . Biomarkers in non-small cell lung cancer prevention. Eur J Cancer Prev 2004; 13: 425–436.
Warner KA, Crawford EL, Zaher A, Coombs RJ, Elsamaloty H, Roshong-Denk SL et al. The c-myc x E2F-1/p21 interactive gene expression index augments cytomorphologic diagnosis of lung cancer in fine-needle aspirate specimens. J Mol Diagn 2003; 5: 176–183.
Shoji T, Tanaka F, Takata T, Yanagihara K, Otake Y, Hanaoka N et al. Clinical significance of p21 expression in non-small-cell lung cancer. J Clin Oncol 2002; 20: 3865–3871.
Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix MJ, Wu R et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol 1997; 17: 353–360.
Hong CF, Chou YT, Lin YS, Wu CW . MAD2B, a novel TCF4-binding protein, modulates TCF4-mediated epithelial-mesenchymal transdifferentiation. J Biol Chem 2009; 14: 14.
Acknowledgements
We thank Dr. Scott Kominsky and Dr. Kristin Webber for their discussion. This research was supported by the Institute of Biomedical Sciences, Academia Sinica, National Yang-Ming University, and the National Science Council (NSC100–2325-B-010–011, NSC100–3112-B-010–004 and NSC100–2321-B-010–021), Executive Yuan, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by BD Dynlacht
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Chou, YT., Hsieh, CH., Chiou, SH. et al. CITED2 functions as a molecular switch of cytokine-induced proliferation and quiescence. Cell Death Differ 19, 2015–2028 (2012). https://doi.org/10.1038/cdd.2012.91
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.91
Keywords
This article is cited by
-
In vivo genome-wide CRISPR screening identifies CITED2 as a driver of prostate cancer bone metastasis
Oncogene (2024)
-
Sex differences in the tumor promoting effects of tobacco smoke in a cRaf transgenic lung cancer disease model
Archives of Toxicology (2024)
-
Critical role of SOX2–IGF2 signaling in aggressiveness of bladder cancer
Scientific Reports (2020)
-
Cited2 regulates proliferation and survival in young and old mouse cardiac stem cells
BMC Molecular and Cell Biology (2019)
-
OCT4B mediates hypoxia-induced cancer dissemination
Oncogene (2019)