Abstract
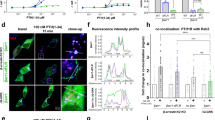
Apoptosis is programmed cell death triggered by activation of death receptors or cellular stress. Activation of caspases is the hallmark of apoptosis. Arrestins are best known for their role in homologous desensitization of G protein-coupled receptors (GPCRs). Arrestins quench G protein activation by binding to activated phosphorylated GPCRs. Recently, arrestins have been shown to regulate multiple signalling pathways in G protein-independent manner via scaffolding signalling proteins. Here we demonstrate that arrestin-2 isoform is cleaved by caspases during apoptosis induced via death receptor activation or by DNA damage at evolutionarily conserved sites in the C-terminus. Caspase-generated arrestin-2-(1-380) fragment translocates to mitochondria increasing cytochrome C release, which is the key checkpoint in cell death. Cells lacking arrestin-2 are significantly more resistant to apoptosis. The expression of wild-type arrestin-2 or its cleavage product arrestin-2-(1-380), but not of its caspase-resistant mutant, restores cell sensitivity to apoptotic stimuli. Arrestin-2-(1-380) action depends on tBID: at physiological concentrations, arrestin-2-(1-380) directly binds tBID and doubles tBID-induced cytochrome C release from isolated mitochondria. Arrestin-2-(1-380) does not facilitate apoptosis in BID knockout cells, whereas its ability to increase caspase-3 activity and facilitate cytochrome C release is rescued when BID expression is restored. Thus, arrestin-2-(1-380) cooperates with another product of caspase activity, tBID, and their concerted action significantly contributes to cell death.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 1-380:
-
arrestin-2-(1-380) fragment generated by caspase cleavage
- A3KO:
-
arrestin-3 knockout
- CHX:
-
cycloheximide
- COX-IV:
-
subunit IV of cytochrome C oxidase
- DblE:
-
arrestin-2-D380E, D408E caspase-resistant mutant
- DKO:
-
arrestin-2/3 double knockout
- FL:
-
full-length
- GPCR:
-
G protein-coupled receptor
- MEF:
-
mouse embryonic fibroblast
- PARP:
-
poly-(ADP-ribose) polymerase
- tBID:
-
caspase-cleaved BID
- TNFα:
-
tumor necrosis factor α
References
Crawford ED, Wells JA . Caspase substrates and cellular remodeling. Annu Rev Biochem 2011; 80: 1055–1087.
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Galluzzi L, Kepp O, Kroemer G . Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13: 780–788.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD . Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol 2012; 10: e1001394.
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR . The BCL-2 family reunion. Mol Cell 2010; 37: 299–310.
Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 2011; 44: 517–531.
Chipuk JE, Green DR . How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 2008; 18: 157–164.
Dix MM, Simon GM, Cravatt BF . Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell 2008; 134: 679–691.
Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, Wells JA . Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell 2008; 134: 866–876.
Wolan DW, Zorn JA, Gray DC, Wells JA . Small-molecule activators of a proenzyme. Science 2009; 326: 853–858.
Gurevich EV, Gurevich VV . Arrestins are ubiquitous regulators of cellular signaling pathways. Genome Biol 2006; 7: 236.
Kantari C, Walczak H . Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 2011; 1813: 558–563.
Wang L, Du F, Wang X . TNF-α induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703.
Timmer JC, Salvesen GS . Caspase substrates. Cell Death Differ 2007; 14: 66–72.
Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM . Distinct caspase cascades are initiated in receptor-medicated and chemical-induced apoptosis. J Biol Chem 1999; 274: 5053–5060.
Lüthi AU, Martin SJ . The CASBAH: a searchable database of caspase substrates. Cell Death Differ 2007; 14: 641–650.
Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C . Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure 2001; 9: 869–880.
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM et al. A unified model for apical caspase activation. Mol Cell 2003; 11: 529–541.
Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS . Activation of caspases-8 and -10 by FLIP(L). Biochem J 2004; 382: 651–657.
Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM . Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol 2005; 175: 3469–3473.
Bohgaki T, Mozo J, Salmena L, Matysiak-Zablocki E, Bohgaki M, Sanchez O et al. Caspase-8 inactivation in T cells increases necroptosis and suppresses autoimmunity in Bim-/- mice. J Cell Biol 2011; 195: 277–291.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000; 1: 489–495.
Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C et al. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol 2008; 9: 1047–1054.
Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P . Tumor necrosis factor-mediated cell death: to break or to burst, that's the question. Cell Mol Life Sci 2010; 67: 1567–1579.
Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV . Visual and both non-visual arrestins in their ‘inactive’ conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem 2006; 281: 21491–21499.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 2000; 14: 2060–2071.
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 2009; 36: 487–499.
Gurevich EV, Benovic JL, Gurevich VV . Arrestin2 expression selectively increases during neural differentiation. J Neurochem 2004; 91: 1404–1416.
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 2006; 8: 1348–1358.
Idziorek T, Estaquier J, De Bels F, Ameisen JC . YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods 1995; 185: 249–258.
Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K . Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 1999; 93: 3053–3063.
de Vries JF, Wammes LJ, Jedema I, van Dreunen L, Nijmeijer BA, Heemskerk MH et al. Involvement of caspase-8 in chemotherapy-induced apoptosis of patient derived leukemia cell lines independent of the death receptor pathway and downstream from mitochondria. Apoptosis 2007; 12: 181–193.
Wieder T, Essmann F, Prokop A, Schmelz K, Schulze-Osthoff K, Beyaert R et al. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood 2001; 97: 1378–1387.
von Haefen C, Wieder T, Essmann F, Schulze-Osthoff K, Dörken B, Daniel PT . Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene 2003; 22: 2236–2247.
Shelton SN, Shawgo ME, Robertson JD . Cleavage of Bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in Jurkat cells. J Biol Chem 2009; 284: 11247–11255.
Köhler B, Anguissola S, Concannon CG, Rehm M, Kögel D, Prehn JH . Bid participates in genotoxic drug-induced apoptosis of HeLa cells and is essential for death receptor ligands' apoptotic and synergistic effects. PLoS One 2008; 3: e2844.
Slee EA, Keogh SA, Martin SJ . Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 2000; 7: 556–565.
Michalak EM, Villunger A, Adams JM, Strasser A . In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 2008; 15: 1019–1029.
Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038.
Gurevich VV, Gurevich EV . The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharm Ther 2006; 110: 465–502.
Lefkowitz RJ, Shenoy SK . Transduction of receptor signals by beta-arrestins. Science 2005; 308: 512–517.
Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R . A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 2000; 28: 139–152.
Alloway PG, Howard L, Dolph PJ . The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 2000; 28: 129–138.
Chen J, Shi G, Concepcion FA, Xie G, Oprian D, Chen J . Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J Neurosci 2006; 26: 11929–11937.
Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem 2003; 278: 6363–6370.
Luan B, Zhang Z, Wu Y, Kang J, Pei G . Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J 2005; 24: 4237–4246.
Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D et al. Critical regulation of CD4(+) T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol 2007; 8: 817–824.
Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ . {beta}-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem 2009; 284: 8855–8865.
DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW . The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA 2000; 97: 11086–11091.
Povsic TJ, Kohout TA, Lefkowitz RJ . Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 2003; 278: 51334–51339.
Revankar CM, Vines CM, Cimino DF, Prossnitz ER . Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem 2004; 279: 24578–24584.
Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A . Molecular basis of the interaction between proapoptotic truncated BID (tBID) protein and mitochondrial carrier homologue 2 (MTCH2) protein: key players in mitochondrial death pathway. J Biol Chem 2012; 287: 15016–15023.
Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol 2010; 12: 553–562.
Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 2010; 330: 1390–1393.
Kurokawa M, Kornbluth S . Caspases and kinases in a death grip. Cell 2009; 138: 838–854.
Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV . Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biol Chem 1999; 274: 6831–6834.
Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ . beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Nat Acad Sci USA 2001; 98: 1601–1606.
Gurevich VV, Benovic JL . Arrestin: mutagenesis, expression, purification, and functional characterization. Methods Enzymol 2000; 315: 422–437.
Waterhouse NJ, Trapani JA . A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Differ 2003; 10: 853–855.
Acknowledgements
We thank Dr. RJ Lefkowitz, Dr. LA Donoso, Dr. AL George, Dr. SS Zinkel, and Dr. SM Hedrick for A3KO and DKO MEFs, F4C1 antibody, pCMS vector, BID-deficient MEFs, and Caspase-8 fl/fl MEFs, respectively. Supported in part by NIH grants NS045117 and NS065868 (EVG), GM077561, GM081756, and EY011500 (VVG), GM047417 (JLB), Vanderbilt University Discovery Grant 1040659012 (VVG), and NARSAD Young Investigator Award (EVG). VUMC Cell Imaging Core was supported by S10 RR015682, and VUMC Flow Cytometry Shared Resource was supported by P30 CA68485 and DK058404.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by B Zhivotovsky
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Kook, S., Zhan, X., Cleghorn, W. et al. Caspase-cleaved arrestin-2 and BID cooperatively facilitate cytochrome C release and cell death. Cell Death Differ 21, 172–184 (2014). https://doi.org/10.1038/cdd.2013.143
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.143
Keywords
This article is cited by
-
Mdm2 enhances ligase activity of parkin and facilitates mitophagy
Scientific Reports (2020)
-
Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer
Scientific Reports (2018)
-
Fas- and Mitochondria-Mediated Signaling Pathway Involved in Osteoblast Apoptosis Induced by AlCl3
Biological Trace Element Research (2018)
-
Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling
Lasers in Medical Science (2018)
-
Nuclear translocation of annexin 1 following oxygen-glucose deprivation–reperfusion induces apoptosis by regulating Bid expression via p53 binding
Cell Death & Disease (2016)