Abstract
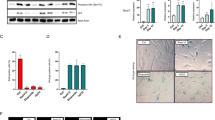
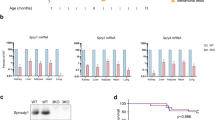
Genes of the Sprouty family (Spry1–4) are feedback inhibitors of receptor tyrosine kinase (RTK) signaling. As such, they restrain proliferation of many cell types and have been proposed as tumor-suppressor genes. Although their most widely accepted target is the Extracellular-regulated kinases (ERK) pathway, the mechanisms by which Spry proteins inhibit RTK signaling are poorly understood. In the present work, we describe a novel mechanism by which Spry1 restricts proliferation, independently of the ERK pathway. In vivo analysis of thyroid glands from Spry1 knockout mice reveals that Spry1 induces a senescence-associated secretory phenotype via activation of the NFκB pathway. Consistently, thyroids from Spry1 knockout mice are bigger and exhibit decreased markers of senescence including Ki67 labeling and senescence-associated β-galactosidase. Although such ‘escape’ from senescence is not sufficient to promote thyroid tumorigenesis in adult mice up to 5 months, the onset of Phosphatase and tensin homolog (Pten)-induced tumor formation is accelerated when Spry1 is concomitantly eliminated. Accordingly, we observe a reduction of SPRY1 levels in human thyroid malignancies when compared with non-tumoral tissue. We propose that Spry1 acts as a sensor of mitogenic activity that not only attenuates RTK signaling but also induces a cellular senescence response to avoid uncontrolled proliferation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ERK:
-
Extracellular-regulated kinases
- MAPK:
-
Mitogen-activated protein kinases
- NFκB:
-
Nuclear Factor κB
- TSH:
-
Thyroid-stimulating hormone
- Pten:
-
Phosphatase and tensin homolog
- STAT3:
-
signal transducer and activator of transcription 3
- IL-6:
-
Interleukin-6
References
Guy GR, Jackson RA, Yusoff P, Chow SY . Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol 2009; 203: 191–202.
Edwin F, Anderson K, Ying C, Patel TB . Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol 2009; 76: 679–691.
McKie AB, Douglas DA, Olijslagers S, Graham J, Omar MM, Heer R et al. Epigenetic inactivation of the human sprouty2 (hSPRY2) homologue in prostate cancer. Oncogene 2005; 24: 2166–2174.
Fritzsche S, Kenzelmann M, Hoffmann MJ, Muller M, Engers R, Grone HJ et al. Concomitant down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr Relat Cancer 2006; 13: 839–849.
Frank MJ, Dawson DW, Bensinger SJ, Hong JS, Knosp WM, Xu L et al. Expression of sprouty2 inhibits B-cell proliferation and is epigenetically silenced in mouse and human B-cell lymphomas. Blood 2009; 113: 2478–2487.
Fong CW, Chua MS, McKie AB, Ling SH, Mason V, Li R et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res 2006; 66: 2048–2058.
Velasco A, Pallares J, Santacana M, Gatius S, Fernandez M, Domingo M et al. Promoter hypermethylation and expression of sprouty 2 in endometrial carcinoma. Hum Pathol 2010; 42: 185–193.
Macià A, Gallel P, Vaquero M, Gou-Fabregas M, Santacana M, Maliszewska A et al. Sprouty1 is a candidate tumor-suppressor gene in medullary thyroid carcinoma. Oncogene 2012; 31: 3961–3972.
Zheng RQ, Abney E, Chu CQ, Field M, Grubeck-Loebenstein B, Maini RN et al. Detection of interleukin-6 and interleukin-1 production in human thyroid epithelial cells by non-radioactive in situ hybridization and immunohistochemical methods. Clin Exp Immunol 1991; 83: 314–319.
Weetman AP, Tandon N, Morgan BP . Antithyroid drugs and release of inflammatory mediators by complement-attacked thyroid cells. Lancet 1992; 340: 633–636.
Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simões M et al. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci USA 2012; 109: E2361–E2370.
Kuilman T, Peeper DS . Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 2009; 9: 81–94.
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL . Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123: 966–972.
Acosta JC, Gil J . Senescence: a new weapon for cancer therapy. Trends Cell Biol 2012; 22: 211–219.
Freund A, Orjalo AV, Desprez PY, Campisi J . Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 2010; 16: 238–246.
Acosta JC, O'Loghlen A, Banito A, Raguz S, Gil J . Control of senescence by CXCR2 and its ligands. Cell Cycle 2008; 7: 2956–2959.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133: 1019–1031.
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6: 2853–2868.
Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR . Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 2008; 132: 363–374.
Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008; 133: 1006–1018.
Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J . Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA 2009; 106: 17031–17036.
Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011; 25: 2125–2136.
Jing H, Kase J, Dörr JR, Milanovic M, Lenze D, Grau M et al. Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev 2011; 25: 2137–2146.
Rovillain E, Mansfield L, Caetano C, Alvarez-Fernandez M, Caballero OL, Medema RH et al. Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene 2011; 30: 2356–2366.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP . Pten is essential for embryonic development and tumour suppression. Nat Genet 1998; 19: 348–355.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 1999; 96: 1563–1568.
Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR . Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/−) mice. Curr Biol 2005; 15: 1839–1846.
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005; 436: 725–730.
Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 2010; 120: 681–693.
de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 2008; 22: 449–462.
Bowman T, Garcia R, Turkson J, Jove R . STATs in oncogenesis. Oncogene 2000; 19: 2474–2488.
Smithgall TE, Briggs SD, Schreiner S, Lerner EC, Cheng H, Wilson MB . Control of myeloid differentiation and survival by Stats. Oncogene 2000; 19: 2612–2618.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Pectasides E, Egloff AM, Sasaki C, Kountourakis P, Burtness B, Fountzilas G et al. Nuclear localization of signal transducer and activator of transcription 3 in head and neck squamous cell carcinoma is associated with a better prognosis. Clin Cancer Res 2010; 16: 2427–2434.
Kim WG, Choi HJ, Kim WB, Kim EY, Yim JH, Kim TY et al. Basal STAT3 activities are negatively correlated with tumor size in papillary thyroid carcinomas. J Endocrinol Invest 2012; 35: 413–418.
Schneller D, Machat G, Sousek A, Proell V, van Zijl F, Zulehner G et al. p19(ARF) /p14(ARF) controls oncogenic functions of signal transducer and activator of transcription 3 in hepatocellular carcinoma. Hepatology 2011; 54: 164–172.
Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S et al. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology 2010; 138: 1003–1011 e1001–e1005.
Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL . Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res 2003; 9: 594–600.
Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005; 436: 720–724.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc Natl Acad Sci USA 1995; 92: 9363–9367.
Campisi J . Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005; 120: 513–522.
Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U . Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 2007; 128: 36–44.
Dumont JE, Lamy F, Roger P, Maenhaut C . Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 1992; 72: 667–697.
Salminen A, Kauppinen A, Kaarniranta K . Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 2012; 24: 835–845.
Burgess DJ . Senescence. NF-κB shows its beneficial side. Nat Rev Cancer 2011; 11: 832–833.
Chiou SH, Shahi P, Wagner RT, Hu H, Lapteva N, Seethammagari M et al. The E3 ligase c-Cbl regulates dendritic cell activation. EMBO Rep 2011; 12: 971–979.
Kometani K, Yamada T, Sasaki Y, Yokosuka T, Saito T, Rajewsky K et al. CIN85 drives B cell responses by linking BCR signals to the canonical NF-kappaB pathway. J Exp Med 2011; 208: 1447–1457.
Liu J, Suresh Kumar KG, Yu D, Molton SA, McMahon M, Herlyn M et al. Oncogenic BRAF regulates beta-Trcp expression and NF-kappaB activity in human melanoma cells. Oncogene 2007; 26: 1954–1958.
Zaremba A, Schmuecker U, Esche H . Sprouty is a cytoplasmic target of adenoviral E1A oncoproteins to regulate the receptor tyrosine kinase signalling pathway. Virol J 2011; 8: 192.
Litovchick L, Florens LA, Swanson SK, Washburn MP, DeCaprio JA . DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev 2011; 25: 801–813.
Schutzman JL, Martin GR . Sprouty genes function in suppression of prostate tumorigenesis. Proc Natl Acad Sci USA 2012; 109: 20023–20028.
Patel R, Gao M, Ahmad I, Fleming J, Singh LB, Rai TS et al. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. J Clin Invest 2013; 123: 1157–1175.
Prieur A, Peeper DS . Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 2008; 20: 150–155.
Collado M, Serrano M . Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 2010; 10: 51–57.
Basolo F, Fiore L, Pollina L, Fontanini G, Conaldi PG, Toniolo A . Reduced expression of interleukin 6 in undifferentiated thyroid carcinoma: in vitro and in vivo studies. Clin Cancer Res 1998; 4: 381–387.
Vizioli MG, Sensi M, Miranda C, Cleris L, Formelli F, Anania MC et al. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene 2010; 29: 3835–3844.
Rodríguez-Rodero S, Fernández AF, Fernández-Morera JL, Castro-Santos P, Bayon GF, Ferrero C et al. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J Clin Endocrinol Metab 2013; 98: 2811–2821.
Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 2006; 10: 459–472.
Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 2010; 151: 4573–4582.
Encinas M, Rozen EJ, Dolcet X, Jain S, Comella JX, Milbrandt J et al. Analysis of Ret knockin mice reveals a critical role for IKKs, but not PI 3-K, in neurotrophic factor-induced survival of sympathetic neurons. Cell Death Differ 2008; 15: 1510–1521.
Acknowledgements
We thank Lidia Parra, Cristina Girón, Sònia Gatius and Maria Santacana for technical support. This work was supported by grants from Ministerio de Economía y Competitividad (BFU2007-67619 and BFU2010-17628) and Fundació La Marató de TV3 (101810), and funding from Suport als Grups de Recerca (2009 SGR 740) from Generalitat de Catalunya to ME, AM is supported by a predoctoral fellowship from Universitat de Lleida and was supported by a fellowship from a Fundació Alícia Cuello de Merigó. MV is supported by a predoctoral fellowship from AGAUR (Generalitat de Catalunya). MG-F is supported by a grant from Fundació La Marató de TV3. CA was supported in part by a Beca de Colaboración (MEC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Deshmukh
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Macià, A., Vaquero, M., Gou-Fàbregas, M. et al. Sprouty1 induces a senescence-associated secretory phenotype by regulating NFκB activity: implications for tumorigenesis. Cell Death Differ 21, 333–343 (2014). https://doi.org/10.1038/cdd.2013.161
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.161
Keywords
This article is cited by
-
Increased SPRY1 expression activates NF-κB signaling and promotes pancreatic cancer progression by recruiting neutrophils and macrophages through CXCL12-CXCR4 axis
Cellular Oncology (2023)
-
Mitochondrial Fus1/Tusc2 and cellular Ca2+ homeostasis: tumor suppressor, anti-inflammatory and anti-aging implications
Cancer Gene Therapy (2022)
-
Regulation of senescence traits by MAPKs
GeroScience (2020)
-
The developing story of Sprouty and cancer
Cancer and Metastasis Reviews (2014)