Abstract
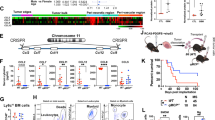
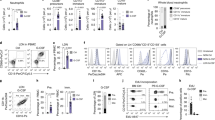
Differentiation of neutrophil granulocytes (neutrophils) occurs through several steps in the bone marrow and requires a coordinate regulation of factors determining survival and lineage-specific development. A number of genes are known whose deficiency disrupts neutrophil generation in humans and in mice. One of the proteins encoded by these genes, glucose-6-phosphatase-β (G6PC3), is involved in glucose metabolism. G6PC3 deficiency causes neutropenia in humans and in mice, linked to enhanced apoptosis and ER stress. We used a model of conditional Hoxb8 expression to test molecular and functional differentiation as well as survival defects in neutrophils from G6PC3−/− mice. Progenitor lines were established and differentiated into neutrophils when Hoxb8 was turned off. G6PC3−/− progenitor cells underwent substantial apoptosis when differentiation was started. Transgenic expression of Bcl-XL rescued survival; however, Bcl-XL-protected differentiated cells showed reduced proliferation, immaturity and functional deficiency such as altered MAP kinase signaling and reduced cytokine secretion. Impaired glucose utilization was found and was associated with ER stress and apoptosis, associated with the upregulation of Bim and Bax; downregulation of Bim protected against apoptosis during differentiation. ER-stress further caused a profound loss of expression and secretion of the main neutrophil product neutrophil elastase during differentiation. Transplantation of wild-type Hoxb8-progenitor cells into irradiated mice allowed differentiation into neutrophils in the bone marrow in vivo. Transplantation of G6PC3−/− cells yielded few mature neutrophils in bone marrow and peripheral blood. Transgenic Bcl-XL permitted differentiation of G6PC3−/− cells in vivo. However, functional deficiencies and differentiation abnormalities remained. Differentiation of macrophages from Hoxb8-dependent progenitors was only slightly disturbed. A combination of defects in differentiation and survival thus underlies neutropenia in G6PC3−/− deficiency, both originating from a reduced ability to utilize glucose. Hoxb8-dependent cells are a model to study differentiation and survival of the neutrophil lineage.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- 2DG:
-
2-deoxy glucose
- BH3 domain:
-
Bcl-2 homology domain 3
- G-CSF:
-
granulocyte colony-stimulating factor
- IL-6:
-
interleukin 6
- LPS:
-
lipopolysaccharide
- Mcl-1:
-
induced myeloid leukemia cell differentiation protein 1
- NE:
-
neutrophil elastase
- SCF:
-
stem cell factor
- SCN:
-
severe congenital neutropenia
- TNF:
-
tumor necrosis factor
- wt:
-
wild type
- GRP-78:
-
glucose-regulated protein 78 kDa
- Hax1:
-
HCLS1 associated protein X-1
- C/EBP:
-
CCAAT/enhancer-binding protein
- CXCR:
-
chemokine (C-X-C motif) receptor
- GFP:
-
green fluorescent protein
References
Klein C . Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol 2011; 29: 399–413.
Kostmann R . Infantile genetic agranulocytosis; agranulocytosis infantilis hereditaria. Acta paediatrica 1956; 45 (Suppl 105): 1–78.
Dale DC, Link DC . The many causes of severe congenital neutropenia. N Engl J Med 2009; 360: 3–5.
Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN . Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature 2008; 452: 98–102.
Cavnar PJ, Berthier E, Beebe DJ, Huttenlocher A . Hax1 regulates neutrophil adhesion and motility through RhoA. J Cell Biol 2011; 193: 465–473.
Kollner I, Sodeik B, Schreek S, Heyn H, von Neuhoff N, Germeshausen M et al. Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood 2006; 108: 493–500.
Grenda DS, Murakami M, Ghatak J, Xia J, Boxer LA, Dale D et al. Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis. Blood 2007; 110: 4179–4187.
Lin B, Annabi B, Hiraiwa H, Pan CJ, Chou JY . Cloning and characterization of cDNAs encoding a candidate glycogen storage disease type 1b protein in rodents. J Biol Chem 1998; 273: 31656–31660.
Guionie O, Clottes E, Stafford K, Burchell A . Identification and characterisation of a new human glucose-6-phosphatase isoform. FEBS Lett 2003; 551: 159–164.
Boztug K, Appaswamy G, Ashikov A, Schaffer AA, Salzer U, Diestelhorst J et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med 2009; 360: 32–43.
Xia J, Bolyard AA, Rodger E, Stein S, Aprikyan AA, Dale DC et al. Prevalence of mutations in ELANE, GFI1, HAX1, SBDS, WAS and G6PC3 in patients with severe congenital neutropenia. Br J Haematol 2009; 147: 535–542.
Arostegui JI, de Toledo JS, Pascal M, Garcia C, Yague J, Diaz de Heredia C . A novel G6PC3 homozygous 1-bp deletion as a cause of severe congenital neutropenia. Blood 2009; 114: 1718–1719.
Banka S, Chervinsky E, Newman WG, Crow YJ, Yeganeh S, Yacobovich J et al. Further delineation of the phenotype of severe congenital neutropenia type 4 due to mutations in G6PC3. Eur J Hum Genet 2011; 19: 18–22.
Cheung YY, Kim SY, Yiu WH, Pan CJ, Jun HS, Ruef RA et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta. J Clin Invest 2007; 117: 784–793.
Jun HS, Lee YM, Cheung YY, McDermott DH, Murphy PM, De Ravin SS et al. Lack of glucose recycling between endoplasmic reticulum and cytoplasm underlies cellular dysfunction in glucose-6-phosphatase-beta-deficient neutrophils in a congenital neutropenia syndrome. Blood 2010; 116: 2783–2792.
Jun HS, Lee YM, Song KD, Mansfield BC, Chou JY . G-CSF improves murine G6PC3-deficient neutrophil function by modulating apoptosis and energy homeostasis. Blood 2011; 117: 3881–3892.
McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P et al. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood 2010; 116: 2793–2802.
Wang GG, Calvo KR, Pasillas MP, Sykes DB, Hacker H, Kamps MP . Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat Methods 2006; 3: 287–293.
Kirschnek S, Vier J, Gautam S, Frankenberg T, Rangelova S, Eitz-Ferrer P et al. Molecular analysis of neutrophil spontaneous apoptosis reveals a strong role for the pro-apoptotic BH3-only protein Noxa. Cell Death Differ 2011; 18: 1805–1814.
Borregaard N . Neutrophils, from marrow to microbes. Immunity 2010; 33: 657–670.
Wang Y, Oeser JK, Yang C, Sarkar S, Hackl SI, Hasty AH et al. Deletion of the gene encoding the ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein (UGRP)/glucose-6-phosphatase catalytic subunit-beta results in lowered plasma cholesterol and elevated glucagon. J Biol Chem 2006; 281: 39982–39989.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A . Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012; 30: 459–489.
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Strasser A . The role of BH3-only proteins in the immune system. Nat Rev Immunol 2005; 5: 189–200.
Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007; 129: 1337–1349.
Hayee B, Antonopoulos A, Murphy EJ, Rahman FZ, Sewell G, Smith BN et al. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology 2011; 21: 914–924.
Schroder M, Kaufman RJ . The mammalian unfolded protein response. Annu Rev Biochem 2005; 74: 739–789.
O'Reilly LA, Huang DC, Strasser A . The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J 1996; 15: 6979–6990.
Jun HS, Cheung YY, Lee YM, Mansfield BC, Chou JY . Glucose-6-phosphatase-beta, implicated in a congenital neutropenia syndrome, is essential for macrophage energy homeostasis and functionality. Blood 2012; 119: 4047–4055.
Pouyssegur J, Shiu RP, Pastan I . Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell 1977; 11: 941–947.
Metcalf D . Hematopoietic cytokines. Blood 2008; 111: 485–491.
Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia 2008; 22: 370–377.
Yamamoto N, Ueda M, Sato T, Kawasaki K, Sawada K, Kawabata K et al. Measurement of Glucose Uptake in Cultured Cells. Current protocols in pharmacology 2011 Chapter 12: Unit 12.14.1-22.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through a grant to GH (SFB 620/A17), and SG was supported by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School). HH was supported by the American Lebanese Syrian Associated Charities (ALSAC). IEG was funded by an EMBO LTF. We thank Dr. Richard O’Brien, Vanderbilt University Medical School, Nashville, for providing bone marrow from G6PC3−/− mice, Dr. Robert Zeiser, Hematology Freiburg, for help with in vivo experiments and Dr. Georg Kochs, Virology, Freiburg, for his generous help with the 2DG assay.
Author Contributions
Experiments were conceived and designed by GH, SG, SK, PH and HH. SG performed most experiments except mRNA analyses (LM and AB) and MAPK analysis (CK). IEG substantially contributed to conducting and analysing the in vivo experiments. The manuscript was written by GH and SG, the figures were made by SG. All authors provided detailed comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Rabinovich
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Gautam, S., Kirschnek, S., Gentle, I. et al. Survival and differentiation defects contribute to neutropenia in glucose-6-phosphatase-β (G6PC3) deficiency in a model of mouse neutrophil granulocyte differentiation. Cell Death Differ 20, 1068–1079 (2013). https://doi.org/10.1038/cdd.2013.39
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.39
Keywords
This article is cited by
-
SLGT2 Inhibitor Rescues Myelopoiesis in G6PC3 Deficiency
Journal of Clinical Immunology (2022)
-
The AML-associated K313 mutation enhances C/EBPα activity by leading to C/EBPα overexpression
Cell Death & Disease (2021)
-
Hypothesis: A Novel Neuroprotective Role for Glucose-6-phosphatase (G6PC3) in Brain—To Maintain Energy-Dependent Functions Including Cognitive Processes
Neurochemical Research (2020)
-
The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling
Cell Death & Disease (2016)
-
Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage
Nature Medicine (2014)