Abstract
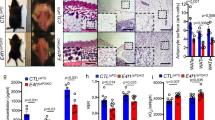
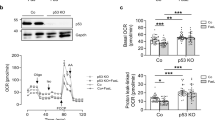
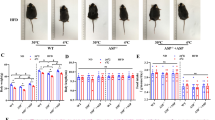
Proper regulation of white and brown adipogenic differentiation is important for maintaining an organism’s metabolic profile in a homeostatic state. The recent observations showing that the p53 tumor suppressor plays a role in metabolism raise the question of whether it is involved in the regulation of white and brown adipocyte differentiation. By using several in vitro models, representing various stages of white adipocyte differentiation, we found that p53 exerts a suppressive effect on white adipocyte differentiation in both mouse and human cells. Moreover, our in vivo analysis indicated that p53 is implicated in protection against diet-induced obesity. In striking contrast, our data shows that p53 exerts a positive regulatory effect on brown adipocyte differentiation. Abrogation of p53 function in skeletal muscle committed cells reduced their capacity to differentiate into brown adipocytes and histological analysis of brown adipose tissue revealed an impaired morphology in both embryonic and adult p53-null mice. Thus, depending on the specific adipogenic differentiation program, p53 may exert a positive or a negative effect. This cell type dependent regulation reflects an additional modality of p53 in maintaining a homeostatic state, not only in the cell, but also in the organism at large.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AdipoQ:
-
Adiponectin
- AP-2:
-
Adipocyte-Fatty-Acid-Binding-Protein
- hASC:
-
human Adipose-derived stem cells
- ATCC:
-
American Type Culture Collection
- BAT:
-
brown adipose tissue
- CEBPa:
-
CCAAT-Enhancer-Binding-Protein-α
- ChIP:
-
chromatin immunoprecipitaion
- Cidea:
-
Cell Death-Inducing DNA Fragmentation Factor-like Effector A
- Elovl3:
-
Elongation of Very Long chain fatty acids gene 3
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- HFD:
-
high fat high sucrose diet
- HPRT:
-
hypoxanthine phosphoribosyltransferase
- KO:
-
knockout
- MEFs:
-
mouse embryonic fibroblasts
- mutp53:
-
p53R172H
- ND:
-
normal chow diet
- PGC-1α:
-
PPARγ Coactivator-1α
- PPARγ:
-
Peroxisome Proliferator-Activated-Receptor-γ
- PRDM16:
-
Positive-Regulatory-Domain-Containing-16
- shRNA:
-
short hairpin RNA
- UCP-1:
-
Uncoupling Protein-1
- WAT:
-
white adipose tissue
- wt:
-
wild type
References
Rosen ED, MacDougald OA . Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–896.
Farmer SR . Molecular determinants of brown adipocyte formation and function. Genes Dev 2008; 22: 1269–1275.
Cristancho AG, Lazar MA . Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011; 12: 722–734.
Lidell ME, Enerback S . Brown adipose tissue--a new role in humans? Nat Rev Endocrinol 2010; 6: 319–325.
Gesta S, Tseng YH, Kahn CR . Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242–256.
Khandekar MJ, Cohen P, Spiegelman BM . Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011; 11: 886–895.
Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 2010; 328: 1158–1161.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Lowell BB, V SS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993; 366: 740–742.
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007; 6: 38–54.
Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 2008; 22: 1397–1409.
Fruhbeck G, Sesma P, Burrell MA . PRDM16: the interconvertible adipo-myocyte switch. Trends Cell Biol 2009; 19: 141–146.
Vousden KH, Ryan KM . p53 and metabolism. Nat Rev Cancer 2009; 9: 691–700.
Vousden KH, Prives C . Blinded by the Light: The Growing Complexity of p53. Cell 2009; 137: 413–431.
Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G . The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol 2011; 12: 259–265.
Tapia N, Scholer HR . p53 connects tumorigenesis and reprogramming to pluripotency. J Exp Med 2010; 207: 2045–2048.
Zhu Y, Prives C . p53 and Metabolism: The GAMT Connection. Mol Cell 2009; 36: 351–352.
Molchadsky A, Rivlin N, Brosh R, Rotter V, Sarig R . p53 is balancing development, differentiation and de-differentiation to assure cancer prevention. Carcinogenesis 2010; 31: 1501–1508.
Molchadsky A, Shats I, Goldfinger N, Pevsner-Fischer M, Olson M, Rinon A et al. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS One 2008; 3: e3707.
Armesilla-Diaz A, Elvira G, Silva A . p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res 2009; 315: 3598–3610.
Yahagi N, Shimano H, Matsuzaka T, Najima Y, Sekiya M, Nakagawa Y et al. p53 Activation in adipocytes of obese mice. J Biol Chem 2003; 278: 25395–25400.
Reznikoff CA, Brankow DW, Heidelberger C . Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 1973; 33: 3231–3238.
Bowers RR, Lane MD . A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 2007; 6: 385–389.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844–848.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Cadwell C, Zambetti GP . The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene 2001; 277: 15–30.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009; 460: 1154–1158.
Hallenborg P, Feddersen S, Madsen L, Kristiansen K . The tumor suppressors pRB and p53 as regulators of adipocyte differentiation and function. Expert Opin Ther Targets 2009; 13: 235–246.
Folgiero V, Migliano E, Tedesco M, Iacovelli S, Bon G, Torre ML et al. Purification and characterization of adipose-derived stem cells from patients with lipoaspirate transplant. Cell Transplant 2010; 19: 1225–1235.
Zhang Y, Bellows CF, Kolonin MG . Adipose tissue-derived progenitor cells and cancer. World J Stem Cells 2010; 2: 103–113.
Martin-Padura I, Gregato G, Marighetti P, Mancuso P, Calleri A, Corsini C et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res 2012; 72: 325–334.
Lidell ME, Enerback S . Brown adipose tissue--a new role in humans? In: Nat Rev Endocrinol 2010; vol. 6: England pp 319–325.
Calle EE, Kaaks R . Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579–591.
Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR . beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem 1999; 274: 34795–34802.
Milyavsky M, Shats I, Erez N, Tang X, Senderovich S, Meerson A et al. Prolonged culture of telomerase-immortalized human fibroblasts leads to a premalignant phenotype. Cancer Res 2003; 63: 7147–7157.
McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY . Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 2006; 103: 18267–18272.
Acknowledgements
This research was supported by a Center of Excellence grant from Flight Attendant Medical Research Institute (FAMRI), Yad Abraham Center for Cancer Diagnosis and Therapy and by EC FP7-INFLACARE number-223151. VR is the incumbent of the Norman and Helen Asher Professorial Chair Cancer Research at the Weizmann institute. ED is the incumbent of the Henry J Leir Professorial Chair. We are grateful to Dr. Ori Brenner for pathological analysis and to Dr. Inbal Biton and Dr. Michael Tzoory for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by K Vousden
Disclaimer
This publication reflects the authors’ views and not necessarily those of the European Community. The EC is not liable for any use that may be made of the information contained herein.
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Molchadsky, A., Ezra, O., Amendola, P. et al. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death Differ 20, 774–783 (2013). https://doi.org/10.1038/cdd.2013.9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.9
Keywords
This article is cited by
-
Regulatory modules of human thermogenic adipocytes: functional genomics of large cohort and Meta-analysis derived marker-genes
BMC Genomics (2021)
-
NADPH levels affect cellular epigenetic state by inhibiting HDAC3–Ncor complex
Nature Metabolism (2021)
-
The multifunctional protein E4F1 links P53 to lipid metabolism in adipocytes
Nature Communications (2021)
-
Frataxin deficiency induces lipid accumulation and affects thermogenesis in brown adipose tissue
Cell Death & Disease (2020)
-
Silencing of functional p53 attenuates NAFLD by promoting HMGB1-related autophagy induction
Hepatology International (2020)