Abstract
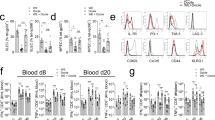
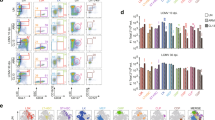
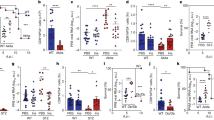
During virus infection and autoimmune disease, inflammatory dendritic cells (iDCs) differentiate from blood monocytes and infiltrate infected tissue. Following acute infection with hepatotropic viruses, iDCs are essential for re-stimulating virus-specific CD8+ T cells and therefore contribute to virus control. Here we used the lymphocytic choriomeningitis virus (LCMV) model system to identify novel signals, which influence the recruitment and activation of iDCs in the liver. We observed that intrinsic expression of Toso (Faim3, FcμR) influenced the differentiation and activation of iDCs in vivo and DCs in vitro. Lack of iDCs in Toso-deficient (Toso–/–) mice reduced CD8+ T-cell function in the liver and resulted in virus persistence. Furthermore, Toso–/– DCs failed to induce autoimmune diabetes in the rat insulin promoter-glycoprotein (RIP-GP) autoimmune diabetes model. In conclusion, we found that Toso has an essential role in the differentiation and maturation of iDCs, a process that is required for the control of persistence-prone virus infection.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ALT:
-
Alanine-amino transferase
- β-ME:
-
Beta-mercaptoethanol
- BMDC:
-
Bone marrow-derived dendritic cell
- FACS:
-
Fluorescence-activated cell sorting
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- GP:
-
Glycoprotein
- IL-6:
-
Interleukin-6
- iDC:
-
inflammatory dendritic cell
- iNOS:
-
inducible nitric oxide synthase
- IFN-γ:
-
Interferon-gamma
- IL7Rα:
-
Interleukin-7 receptor alpha chain
- iMATE:
-
Intrahepatic myeloid cell aggregate
- LPS:
-
Lipopolysaccharide
- LCMV:
-
Lymphocytic choriomeningitis virus
- LCMV-GP:
-
LCMV glycoprotein
- LCMV-GP33:
-
LCMV glycoprotein 33–41
- MFI:
-
Mean fluorescence intensity
- MHC:
-
Major histocompatibility complex
- NF-κB:
-
Nuclear factor ‘kappa-light-chain-enhancer’ of activated B cells
- PD-1:
-
Programmed cell death-1
- RIP:
-
Rat insulin promoter
- PFU:
-
Plaque-forming unit
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- Tip-DCs:
-
TNF-α and iNOS producing DCs
- WT:
-
Wild type
References
Rehermann B, Nascimbeni M . Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5: 215–229.
Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S et al. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol 2002; 36: 105–115.
Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 2001; 194: 1395–1406.
Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med 1995; 181: 1047–1058.
Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T et al. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol 1990; 145: 3442–3449.
Recher M, Lang KS, Navarini A, Hunziker L, Lang PA, Fink K et al. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat Med 2007; 13: 1316–1323.
Geissmann F, Jung S, Littman DR . Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82.
Auffray C, Sieweke MH, Geissmann F . Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009; 27: 669–692.
Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 2009; 10: 394–402.
Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F et al. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog 6: e1001045.
Aldridge JR Jr., Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA 2009; 106: 5306–5311.
Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG . TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19: 59–70.
Serbina NV, Jia T, Hohl TM, Pamer EG . Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26: 421–452.
Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2001; 98: 7958–7963.
Pamer EG . Tipping the balance in favor of protective immunity during influenza virus infection. Proc Natl Acad Sci USA 2009; 106: 4961–4962.
Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013; 14: 574–583.
Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity 1998; 8: 461–471.
Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood 2008; 112: 4213–4219.
Proto-Siqueira R, Panepucci RA, Careta FP, Lee A, Clear A, Morris K et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood 2008; 112: 394–397.
Sigruener A, Buechler C, Bared SM, Grandl M, Aslanidis C, Ugocsai P et al. E-LDL upregulates TOSO expression and enhances the survival of human macrophages. Biochem Biophys Res Commun 2007; 359: 723–728.
Song Y, Jacob CO . The mouse cell surface protein TOSO regulates Fas/Fas ligand-induced apoptosis through its binding to Fas-associated death domain. J Biol Chem 2005; 280: 9618–9626.
Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol 22: 149–156.
Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med 2009; 206: 2779–2793.
Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Föger N et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood 2011; 118: 598–608.
Nguyen X, Lang PA, Lang KS, Adam D, Fattakhova G, Föger N et al. Toso regulates death receptor-induced apoptosis by facilitating RIP1 ubiquitination. Blood 2010; 118 (3): 598–608.
Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA 2013; 110: 2593–2598.
Lang KS, Lang PA, Meryk A, Pandyra AA, Merches K, Lee KH et al. Reply to Honjo et al.: Functional relevant expression of Toso on granulocytes. Proc Natl Acad Sci USA 2013; 110: E2542–E2543.
Brenner D, Brüstle A, Lin GH, Lang PA, Duncan GS, Knobbe-Thomsen CB et al. Toso controls encephalitogenic immune responses by dendritic cells and regulatory T cells. Proc Natl Acad Sci USA 2014; 111: 1060–1065.
Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B et al. Ablation of ‘‘tolerance’’ and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 1991; 65: 305–317.
Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 2005; 11: 138–145.
Honke N et al. Usp18 driven enforced viral replication in dendritic cells contributes to break of immunological tolerance in autoimmune diabetes. PLoS pathog 2013; 9: e1003650.
Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 2012; 13: 51–57.
Ohashi PS, Pircher H, Mak TW, Bürki K, Zinkernagel RM, Hengartner H et al. Ontogeny and selection of the T cell repertoire in transgenic mice. Semin Immunol 1989; 1: 95–104.
Cui W, Kaech SM . Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev 2010; 236: 151–166.
Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 1997; 94: 12053–12058.
Woltman AM, Boonstra A, Janssen HL . Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels? Gut 59: 115–125.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–879.
Frieman M, Heise M, Baric R . SARS coronavirus and innate immunity. Virus Res 2008; 133: 101–112.
Peiris JS, Cheung CY, Leung CY, Nicholls JM . Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol 2009; 30: 574–584.
Lang PA, Recher M, Haussinger D, Lang KS . Genes determining the course of virus persistence in the liver: lessons from murine infection with lymphocytic choriomeningitis virus. Cell Physiol Biochem 2010; 26: 263–272.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687.
Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol 2005; 35: 738–745.
Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R . Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA 2004; 101: 16004–16009.
Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443: 350–354.
Radziewicz H, Hanson HL, Ahmed R, Grakoui A . Unraveling the role of PD-1/PD-L interactions in persistent hepatotropic infections: potential for therapeutic application? Gastroenterology 2008; 134: 2168–2171.
Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol 2010; 22: 149–156.
Vire B, David A, Wiestner A . TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol 2011; 187: 4040–4050.
Nguyen XH, Fattakhova G, Lang PA, Lang KS, Adam D, Föger N et al. Antiapoptotic function of Toso (Faim3) in death receptor signaling. Blood 119: 1790–1791 2012.
Lang KS, Recher M, Navarini AA, Freigang S, Harris NL, van den Broek M et al. Requirement for neutralizing antibodies to control bone marrow transplantation-associated persistent viral infection and to reduce immunopathology. J Immunol 2005; 175: 5524–5531.
Acknowledgements
This study was supported by the Alexander von Humboldt Foundation (SKA2008 to KSL and SKA2010 to PAL). Furthermore, this study was supported by the German Research Council (TRR60, CRC974, LA2558-3-5/1, LA1419/5-1). This work was also supported by CIHR. DB was supported by a postdoctoral fellowship from the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Lang, P., Meryk, A., Pandyra, A. et al. Toso regulates differentiation and activation of inflammatory dendritic cells during persistence-prone virus infection. Cell Death Differ 22, 164–173 (2015). https://doi.org/10.1038/cdd.2014.138
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.138
This article is cited by
-
A porcine reproductive and respiratory syndrome virus (PRRSV)-specific IgM as a novel adjuvant for an inactivated PRRSV vaccine improves protection efficiency and enhances cell-mediated immunity against heterologous PRRSV challenge
Veterinary Research (2022)
-
Fcmr regulates mononuclear phagocyte control of anti-tumor immunity
Nature Communications (2019)
-
The IgM receptor FcμR limits tonic BCR signaling by regulating expression of the IgM BCR
Nature Immunology (2017)