Abstract
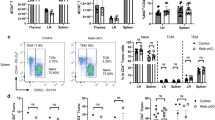
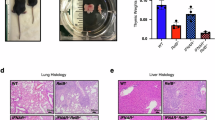
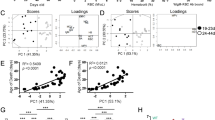
FASL/FAS signaling imposes a critical barrier against autoimmune disease and lymphadenopathy. Mutant mice unable to produce membrane-bound FASL (FasLΔm/Δm), a prerequisite for FAS-induced apoptosis, develop lymphadenopathy and systemic autoimmune disease with immune complex-mediated glomerulonephritis. Prior to disease onset, FasLΔm/Δm mice contain abnormally high numbers of leukocytes displaying activated and elevated NF-κB-regulated cytokine levels, indicating that NF-κB-dependent inflammation may be a key pathological driver in this multifaceted autoimmune disease. We tested this hypothesis by genetically impairing canonical or non-canonical NF-κB signaling in FasLΔm/Δm mice by deleting the c-Rel or NF-κB2 genes, respectively. Although the loss of NF-κB2 reduced the levels of inflammatory cytokines and autoantibodies, the impact on animal survival was minor due to substantially accelerated and exacerbated lymphoproliferative disease. In contrast, a marked increase in lifespan resulting from the loss of c-REL coincided with a striking reduction in classical parameters of autoimmune pathology, including the levels of cytokines and antinuclear autoantibodies. Notably, the decrease in regulatory T-cell numbers associated with loss of c-REL did not exacerbate autoimmunity in FasLΔm/Δmc-rel−/− mice. These findings indicate that selective inhibition of c-REL may be an attractive strategy for the treatment of autoimmune pathologies driven by defects in FASL/FAS signaling that would be expected to circumvent many of the complications caused by pan-NF-κB inhibition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ANA:
-
antinuclear autoantibodies
- GN:
-
glomerulonephritis
- sFASL:
-
soluble FASL
- mFASL:
-
membrane-bound FASL
- mTEC:
-
medullary thymic epithelial cells
- AIRE:
-
autoimmune regulator
References
Strasser A, Harris AW, Cory S . Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 1991; 67: 889–899.
Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG . Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 2005; 435: 590–597.
Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 2002; 415: 922–926.
Strasser A, Jost PJ, Nagata S . The many roles of FAS receptor signaling in the immune system. Immunity 2009; 30: 180–192.
Hughes PD, Belz GT, Fortner K, Budd RC, Strasser A, Bouillet P . Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 2008; 28: 197–205.
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S . Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992; 356: 314–317.
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middelton LA, Lin AY et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995; 81: 935–946.
O'Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 2009; 461: 659–663.
Krammer PH . CD95's deadly mission in the immune system. Nature 2000; 407: 789–795.
Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J et al. Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem 2001; 276: 47100–47106.
Karin M, Lin A . NF-κB at the crossroads of life and death. Nat Immunol 2002; 3: 221–227.
Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W et al. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene 2006; 25: 6781–6799.
Hayden MS, Ghosh S . Shared principles in NF-kappaB signaling. Cell 2008; 132: 344–362.
Gilmore TD . Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006; 25: 6680–6684.
Vallabhapurapu S, Karin M . Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009; 27: 693–733.
Brown KD, Claudio E, Siebenlist U . The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther 2008; 10: 212.
Pai S, Thomas R . Immune deficiency or hyperactivity-Nf-kappab illuminates autoimmunity. J Autoimmun 2008; 31: 245–251.
Zhang B, Wang Z, Ding J, Peterson P, Gunning WT, Ding HF . NF-kappaB2 is required for the control of autoimmunity by regulating the development of medullary thymic epithelial cells. J Biol Chem 2006; 281: 38617–38624.
Siebenlist U, Brown K, Claudio E . Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol 2005; 5: 435–445.
Flammer JR, Rogatsky I . Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol 2011; 25: 1075–1086.
Grumont RJ, Gerondakis S . The murine c-rel proto-oncogene encodes two mRNAs the expression of which is modulated by lymphoid stimuli. Oncogene Res 1990; 5: 245–254.
Gerondakis S, Strasser A, Metcalf D, Grigoriadis G, Scheerlinck J-PY, Grumont RJ . Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA 1996; 93: 3405–3409.
Gilmore TD, Gerondakis S . The c-Rel transcription factor in development and disease. Genes Cancer 2011; 2: 695–711.
Caamaño JH, Rizzo CA, Durham SK, Barton DS, Raventós-Suárez C, Snapper CM et al. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med 998; 87: 85–96.
Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A et al. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med 998; 87: 47–59.
Zhu M, Chin RK, Christiansen PA, Lo JC, Liu X, Ware C et al. NF-kappaB2 is required for the establishment of central tolerance through an Aire-dependent pathway. J Clin Invest 2006; 116: 2964–2971.
Braun D, Geraldes P, Demengeot J . Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun 2003; 20: 15–25.
Köntgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev 1995; 9: 1965–1977.
Werfel T . The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol 2009; 129: 1878–1891.
Wing K, Sakaguchi S . Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 2010; 11: 7–13.
Akdis M, Blaser K, Akdis CA . T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol 2005; 116: 961–968 quiz 969.
Fyhrquist N, Lehtimaki S, Lahl K, Savinko T, Lappetelainen AM, Sparwasser T et al. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J Invest Dermatol 2012; 132: 1672–1680.
Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206: 3001–3014.
Mathis D, Benoist C . Aire. Annu Rev Immunol 2009; 27: 287–312.
Gray DH, Gavanescu I, Benoist C, Mathis D . Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci USA 2007; 104: 18193–18198.
Irla M, Hollander G, Reith W . Control of central self-tolerance induction by autoreactive CD4(+) thymocytes. Trends Immunol 2010; 31: 71–79.
Strnad J, Burke JR . IkappaB kinase inhibitors for treating autoimmune and inflammatory disorders: potential and challenges. Trends Pharmacol Sci 2007; 28: 142–148.
Vallabhapurapu S, Ryseck RP, Malewicz M, Weih DS, Weih F . Inhibition of NF-κB in T cells blocks lymphoproliferation and partially rescues autoimmune disease in gld/gld mice. Eur J Immunol 2001; 31: 2612–2622.
Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity 2008; 29: 615–627.
Basak S, Kim H, Kearns JD, Tergaonkar V, O'Dea E, Werner SL et al. A fourth IkappaB protein within the NF-kappaB signaling module. Cell 2007; 128: 369–381.
Ishimaru N, Kishimoto H, Hayashi Y, Sprent J . Regulation of naive T cell function by the NF-kappaB2 pathway. Nat Immunol 2006; 7: 763–772.
Brady K, Fitzgerald S, Moynagh PN . Tumour-necrosis-factor-receptor-associated factor 6, NF-kappaB-inducing kinase and IkappaB kinases mediate IgE isotype switching in response to CD40. Biochem J 2000; 350: 735–740.
Nakamura H, Aoki M, Tamai K, Oishi M, Ogihara T, Kaneda Y et al. Prevention and regression of atopic dermatitis by ointment containing NF-kB decoy oligodeoxynucleotides in NC/Nga atopic mouse model. Gene Ther 2002; 9: 1221–1229.
Zelazowski P, Shen Y, Snapper CM . NF-kappaB/p50 and NF-kappaB/c-Rel differentially regulate the activity of the 3'alphaE-hsl,2 enhancer in normal murine B cells in an activation-dependent manner. Int Immunol 2000; 12: 1167–1172.
Carrasco D, Cheng J, Lewin A, Warr G, Yang H, Rizzo C et al. Multiple hemopoietic defects and lymphoid hyperplasia in mice lacking the transcriptional activation domain of the c-Rel protein. J Exp Med 1998; 187: 973–984.
Campbell IK, Gerondakis S, O'Donnell K, Wicks IP . Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest 2000; 105: 1799–1806.
Lamhamedi-Cherradi SE, Zheng S, Hilliard BA, Xu L, Sun J, Alsheadat S et al. Transcriptional regulation of type I diabetes by NF-kappa B. J Immunol 2003; 171: 4886–4892.
Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S et al. The NF-kappaB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol 2011; 187: 4483–4491.
Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest 2002; 110: 843–850.
Chang M, Jin W, Chang JH, Xiao Y, Brittain GC, Yu J et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat Immunol 2011; 12: 1002–1009.
Goodnow CC . Multistep pathogenesis of autoimmune disease. Cell 2007; 130: 25–35.
Gray DH, Chidgey AP, Boyd RL . Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods 2002; 260: 15–28.
Gray DH, Fletcher AL, Hammett M, Seach N, Ueno T, Young LF et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods 2008; 329: 56–66.
Seach N, Wong K, Hammett M, Boyd RL, Chidgey AP . Purified enzymes improve isolation and characterization of the adult thymic epithelium. J Immunol Methods 2012; 385: 23–34.
Naik S, Vremec D, Wu L, O'Keeffe M, Shortman K . CD8alpha+ mouse spleen dendritic cells do not originate from the CD8alpha- dendritic cell subset. Blood 2003; 102: 601–604.
Acknowledgements
We thank G Siciliano, K Hughes, J Coughlin, K McKenzie and F Dabrowski, for animal care; J Corbin for automated blood analysis; B Helbert and C Young for genotyping; and E Tsui, V Babo, K Weston and all histology staff for preparation of histological sections. This work was supported by fellowships and grants from the NHMRC (Canberra; programs #461221 and #1016701, fellowship; (DG) #637353 and project grants #1009145 (LOR), #637332 (DG) and an NHMRC infrastructure grant, Independent Research Institutes Infrastructure Support Scheme Grant #361646, the Victorian State Government (OIS grant), the Leukemia and Lymphoma Society (SCOR grant #7413 and #7001-13) and the JDRF/NHMRC #466658 (AS). This research was also supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (US).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
O'Reilly, L., Hughes, P., Lin, A. et al. Loss of c-REL but not NF-κB2 prevents autoimmune disease driven by FasL mutation. Cell Death Differ 22, 767–778 (2015). https://doi.org/10.1038/cdd.2014.168
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2014.168
This article is cited by
-
Regulation of B-cell function by NF-kappaB c-Rel in health and disease
Cellular and Molecular Life Sciences (2020)
-
Impact of loss of NF‐κB1, NF‐κB2 or c‐REL on SLE‐like autoimmune disease and lymphadenopathy in Faslpr/lpr mutant mice
Immunology & Cell Biology (2016)
-
Linear ubiquitin chain assembly complex coordinates late thymic T-cell differentiation and regulatory T-cell homeostasis
Nature Communications (2016)